The alloy catalyzes CO2 to CH4, and the conversion rate is 98%!
A series of environmental problems have been caused by a large amount of CO2 emissions generated during the utilization of fossil fuels. Driven by the rapid development of green hydrogen and CO2 capture technology, producing hydrocarbon fuels and chemicals through CO2 hydrogenation is becoming an effective process to reduce carbon footprint and store renewable energy. Among them, methanation of CO2 can not only consume CO2, but also store H2 with high density. However, due to the inert nature of CO2 molecules, catalytic conversion under mild conditions is extremely challenging.
It is expected to realize CO2 conversion under mild conditions by photothermal catalysis, and the LSPR effect based on precious metals has been well applied to catalyze this kind of reaction. However, the development of non-noble metal-based catalysts with LSPR effect is still challenging and promising. In recent years, layered double hydroxides (LDHs) containing readily available transition metals have been proved to be a promising raw material for CO2 photothermal reduction due to their unique layered structure, flexible chemical composition, customizable morphology, low cost and high metal dispersibility.
Recently, a team of professors Cheng Kang and Wang Ye from Xiamen University designed a series of non-precious metal NiFeM (M stands for different types of promoting metals) catalysts for photothermal CO2 methanation. NiFe nano-alloy catalyst has been proved to have excellent activity in photothermal heating and LSPR enhancement. The key performance determinants such as structure promoter, light absorption efficiency, NiFe particle size and Ni/Fe ratio were studied and clarified. The optimized NiFeAl has unique flower-like morphology and highly dispersed NiFeAl alloy nanoparticles. Even under room temperature light irradiation, the CO2 conversion rate is 98% and the CH4 selectivity is as high as 99%. In addition, the catalyst is stable within 100 h and has great application potential.
Compared with other catalysts prepared, it is found that the smaller alloy particle size (~21 nm) and unique nano-flower structure in NiFeAl catalyst promote the LSPR enhancement effect of NiFe alloy. At the same time, compared with single metal, Ni and Fe in NiFe alloy can synergistically promote CO2 methanation. Using infrared camera, the surface temperature of the catalyst can be detected as high as 356°C under illumination, which shows that the catalyst can effectively convert light energy into heat energy and provide enough energy for the reaction. This work not only prepared an efficient catalyst for CO2 methanation, but also provided an idea for the structural design of photothermal catalyst.
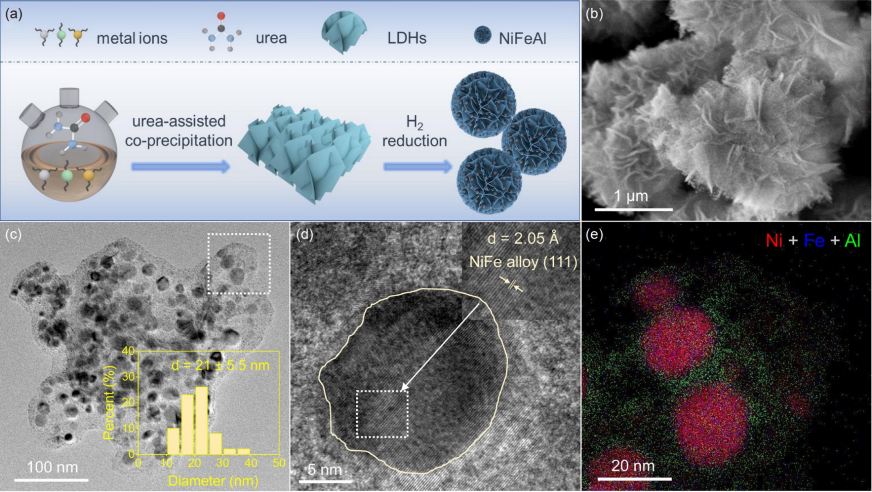
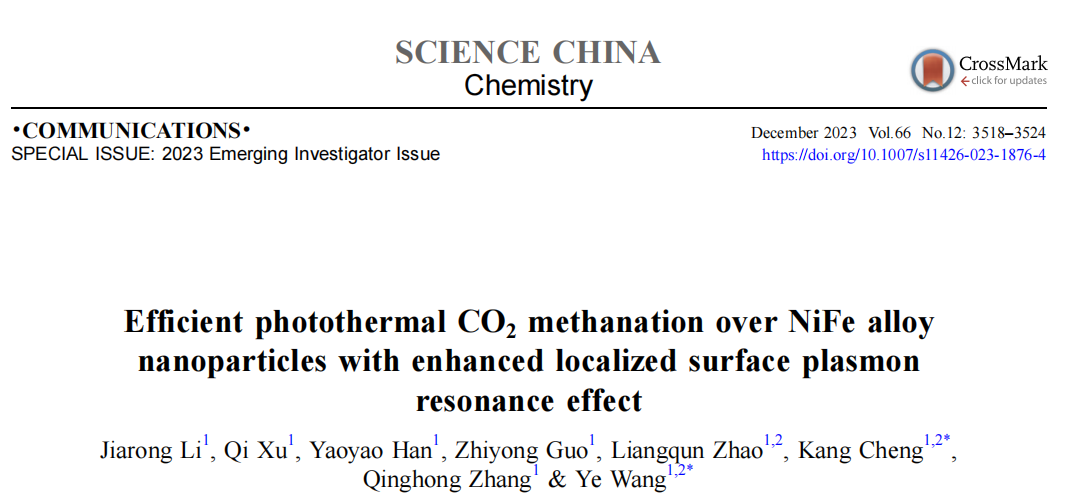
Fig. 1: (a) Process scheme of catalyst preparation. (b) SEM imaging of NiFeAl. TEM (c-e), HR-TEM and STEM-EDX images (color online) of the reduced NiFeAl catalyst.
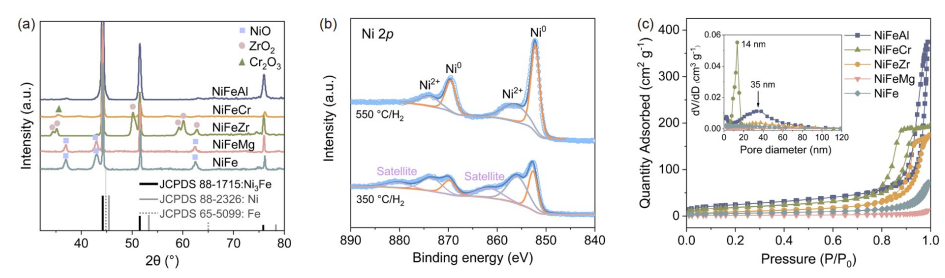
Fig. 2 (a) XRD spectrum of NiFEM catalyst. (b) In-situ high-resolution Ni 2p XPS spectra of NiFeAl reduced by H2 at 350℃ and 550℃. (c) N2 physical adsorption isotherm and BJH pore size distribution curve on NiFEM catalyst (color online).
![]()
Fig. 3 (a) Schematic diagram of CO2 conversion photothermal reactor. The methanation of CO2 was carried out on NiFeM catalyst by (b) thermal catalytic process (350 C/dark) and (c) photothermal process (light radiation). Reaction conditions: catalyst 200 mg, 300 W xenon lamp as light source, H2/CO2 = 3:1, F =20 mL min-1, P = 40 bar (online coloring).
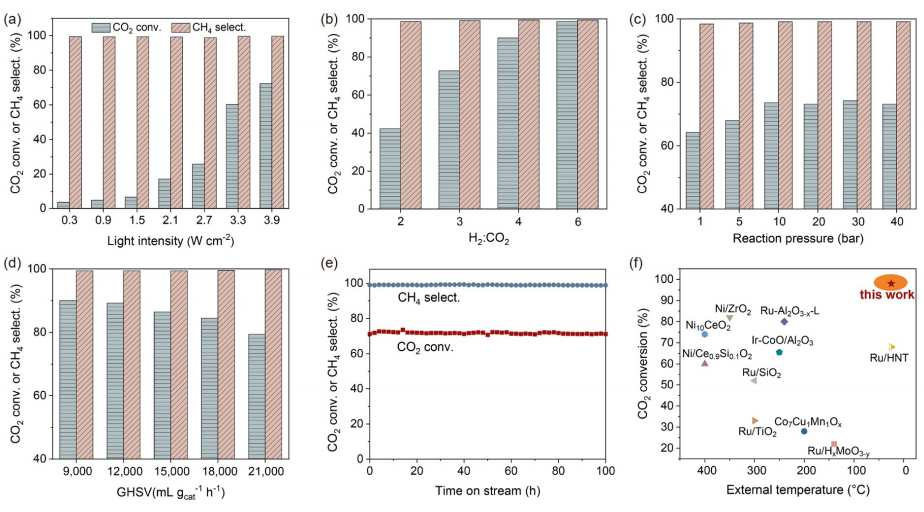
Fig. 4 Effects of kinetic parameters on the methanation performance of photothermal CO2 without external heating: (a) light intensity, (b) H2/CO2 ratio, (c) reaction pressure, and (d) gas hourly space velocity (GHSV). (e) catalyst stability. (f) Comparison between NiFeAl and reported catalysts in photo-thermal CO2 methanation. All reaction conditions and catalytic results are listed in Table S3 and Table S4 (color online).
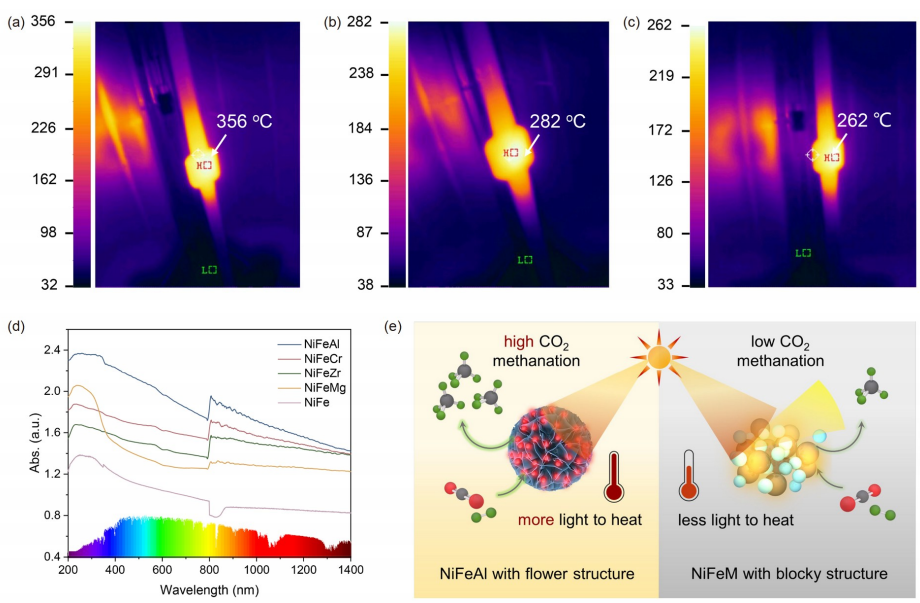
Fig. 5 (a) NiFeAl, (b) nifeecr and (c) NiFeZr infrared camera detected temperature distribution. (d) Ultraviolet-visible absorption spectrum of NiFEM catalyst. (e) Schematic diagram of photo-thermal CO2 methanation process of Ni-Fe-based catalysts with different nanostructures (online color).
Source:https://mp.weixin.qq.com/s/mF1zHD614iJCpF6cqa4CIw
