Preparation of Fe-Co Bimetallic Catalyst and Study on the Performance of CO2 Hydrogenation

It is of great significance to adjust the distribution of CO2 hydrogenation products to obtain high selectivity target products. However, due to the inaccurate regulation of chain propagation and hydrogenation reaction, it is challenging to synthesize a single product directionally. Here, the author reports a method of controlling multiple sites by using graphene fence engineering, which can directly convert CO2/H2 mixture into different types of hydrocarbons. The selectivity of Fe-Co active sites on the surface of graphene fence to light olefins is 50.1%, while the selectivity of space Fe-Co nanoparticles separated by graphene fence to liquefied petroleum gas is 43.6%. With the help of graphene fence, iron carbide and cobalt can effectively regulate the C-C coupling and the second hydrogenation reaction of olefins, and realize the product selective switching between light olefins and liquefied petroleum gas. In addition, compared with other reported composite catalysts, it also pioneered the direct hydrogenation of carbon dioxide to liquefied petroleum gas through Fischer-Tropsch route, with the highest space-time yield.
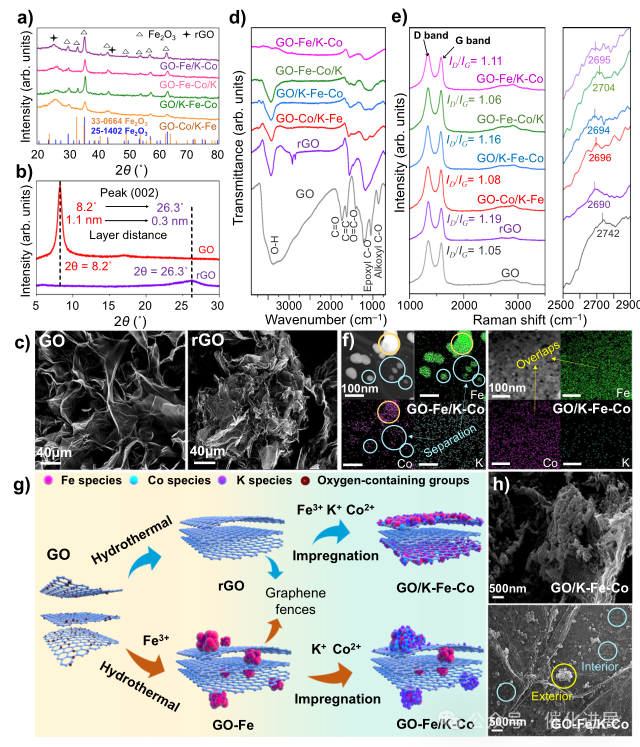
Fig. 1 Study on the structure and morphology of FeCo catalyst supported on graphene
By changing the addition order of Fe and Co during hydrothermal and impregnation, the author synthesized a series of graphene-supported Fe-Co bimetallic catalysts with the same total Fe, Co and K contents (supplementary figure 3).
In order to prove the dynamic evolution process, the author used in-situ XRD to detect the diffraction peak shift of GO in the process of temperature programmed reduction. With the increase of temperature and the introduction of H2, the diffraction peak gradually moves to a higher direction (Supplementary Figure 4), which represents the decrease of the spacing between graphene layers, which is determined by Bragg's law. Similarly, fig. 1b shows that the reduced graphene oxide obtained by hydrothermal treatment also shows higher peaks and smaller interlayer spacing than graphene oxide. At the same time, the specific surface area decreased significantly (Supplementary Table 2). These phenomena corresponding to the folding and bending of graphene layers can be observed from the SEM image of fig. 1c.
In fig. 1d, the author uses FTIR spectra to identify the loss of oxygen-containing groups substituted by metal sites. O−H (3400 cm-1), C = O (1722 cm-1), aromatic C = C (1620 cm-1), carboxyl O = C−O (1356 cm-1), epoxy C−O (1220 cm-1) and alkoxy C−O (1050 cm-1) recorded as reference. After hydrothermal synthesis, the oxygen-containing group peak of reduced graphene oxide is obviously weakened, and two broad peaks at 1550 cm-1 and 1175 cm-1 appear, which belong to C = C and C-O respectively. Interestingly, compared with the reduction of graphene oxide, the vibration peaks of metal-supported catalysts show smaller areas, especially in GO-Fe/K- Co and GO-Fe- Co /K, which can be explained by the fact that metals replace more oxygen-containing groups during the reduction process, which is caused by the metal loading on the inner layer of graphene. However, due to the protection of graphene fence, the metals in GO/ K-Fe- Co and GO- Co /K-Fe can not fully replace the oxygen-containing groups in the layer, resulting in a larger peak area.
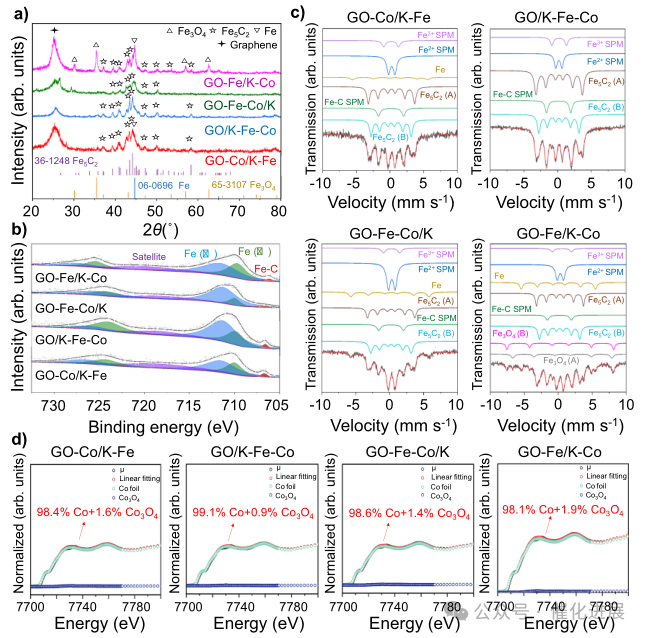
Fig. 2 Phase composition of graphene supported Fe-Co bimetallic catalyst
For the spent catalyst, the fe2p spectrum in Figure 2b shows a binding energy peak of 708.5 eV, which belongs to Fe- c bond, indicating the existence of iron carbide. According to the result of XRD pattern in fig. 2a, the diffraction peak of Fe5C2 was observed in all the spent catalysts. The diffraction peak with the strongest intensity corresponds to the crystal plane of Fe5C2(510), and the HR-TEM image with a lattice spacing of 0.20 nm also shows the crystal plane of Fe5C2(510).
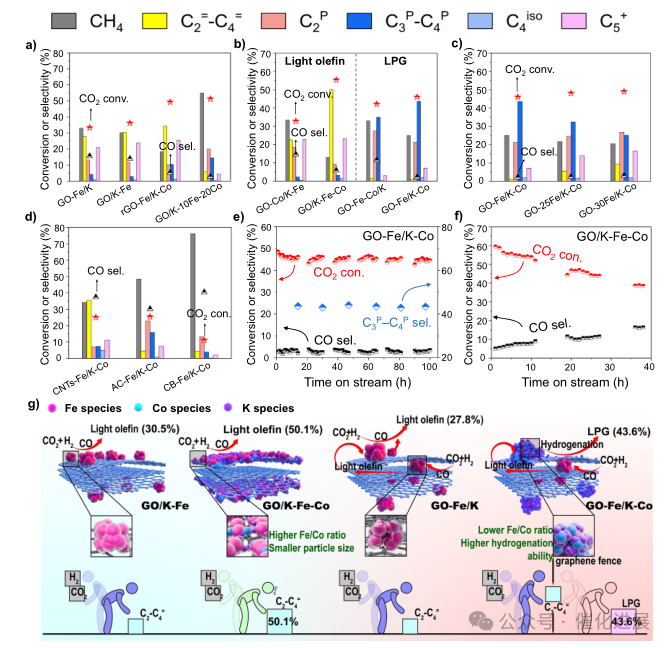
Fig. 3 Catalytic performance and stability
The main products of GO- fe /K and GO/KFe catalysts in Figure 3a are methane and light olefins, not saturated light paraffin, without adding CO.. However, as shown in fig. 3b, with the addition of Co, the Fe-Co bimetallic catalyst shows different types of hydrocarbon product selectivity. GO- co /K-Fe and GO/K-Fe- co mainly produce light olefins, especially the C2 = -C4 = selectivity of GO/KFe-Co catalyst is 50.1%, and the CO2 conversion rate is 55.4%. For GO-Fe-Co/K, more alkanes are produced, especially methane and ethane (fig. 3b). However, compared with GO-Fe- co /K, GO-Fe/K- co has higher selectivity for propane and butane (43.6%) (Figure 3b).
High methane selectivity proves that the external Cofe site with high co content has strong hydrogenation ability (fig. 3a), which is beneficial to the hydrogenation of the original light olefins produced at the internal Fe site to saturated alkanes as shown in fig. 3g, thus converting the products from light olefins to LPG. In this process, the reaction equilibrium moved to the positive direction, which led to the increase of CO2 conversion from 33.2% (GO-Fe/K) to 46.0% (GO-Fe/K- co). As shown in fig. 3g, CO2 hydrogenation is a structurally sensitive reaction, and higher dispersion is beneficial to improve the carbonization ability of Fe, thus improving the catalytic performance. It can be seen from the Fe Mössbauer spectrum of the waste catalyst (Figure 2c and Supplementary Table 8) that the proportion of Fe5C2 in GO/K-Fe-Co is the largest (72%), including Fe5C2 (A) and Fe5C2 (B), so the proportion of Fe5C2 to CO2 conversion (55.4%) in GO/K-Fe-Co is the highest and it has quite high light.
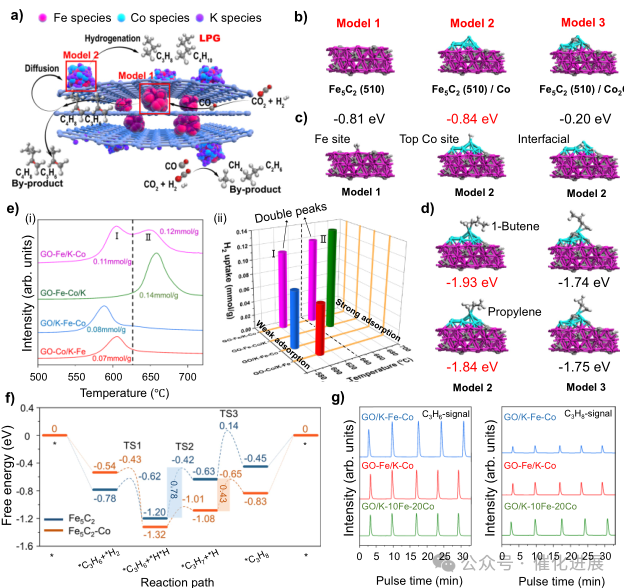
Fig. 4 Exploration of reaction mechanism and pathway
According to the previously reported local microenvironment of supported metal catalysts, in this study, the author used density functional theory (DFT) calculation to explore the influence of Fe-Co double position separated by graphene fence. In order to accurately identify the microenvironment of each active site, the author first constructed a surface model of Fe5C2(510), which was named as Model 1.
Then, as shown in fig. 4b, considering the doping of cobalt on the surface of GO-Fe/K-Co, Co10 cluster was added to Model 1 to construct Model 2. Among them, Model 1 corresponds to the active site of Fe5C2 in the GO-Fe/K-Co graphene fence, and Model 2 corresponds to the active site of Fe5C2/Co on the surface of the graphene fence (Figure 4b). In order to compare the adsorption capacity of carbonized Co and metal Co, Co10 in Model 2 was replaced by Co8C4, which was called "Model 3" (Figure 4b).
By analyzing the reaction results and mechanism characterization, the reaction path of selective CO2 hydrogenation over GO-Fe/K-Co catalyst is proposed as shown in Figure 4a. Firstly, CO2 is converted into CO by RWGS reaction at the internal Fe active site, and then light olefins are produced by FTS process. Diffusion effect can easily transfer light olefins to CoFe active sites on the outer surface of graphene fences, and the cobalt content of CoFe active sites is relatively high. Because olefins have high hydrogen adsorption capacity, olefins are hydrogenated to light alkanes, resulting in highly selective liquefied petroleum gas. Notably, the adsorption intensity of propylene and 1- butene on the surface of metal Co in Model 2 is stronger than that on Co2C in Model 3 (Figure 4d), which proves that the olefin hydrogenation efficiency of metal Co to Co2C is higher in this unique CO2 hydrogenation reaction system. At the same time, due to the inactivity of Co in RWGS reaction, the Co loaded on the outer surface will consume a large amount of Co produced by the internal Fe active sites, resulting in a very low CO selectivity (2.2%). However, due to the lack of RWGS reaction, the high concentration of H2 forced CO2 to be directly hydrogenated to methane and ethane at the site of Fe5C2/Co on the outer surface of graphene fence, which was proved by the catalytic performance of GO/K-10Fe-20Co (Figure 3a).
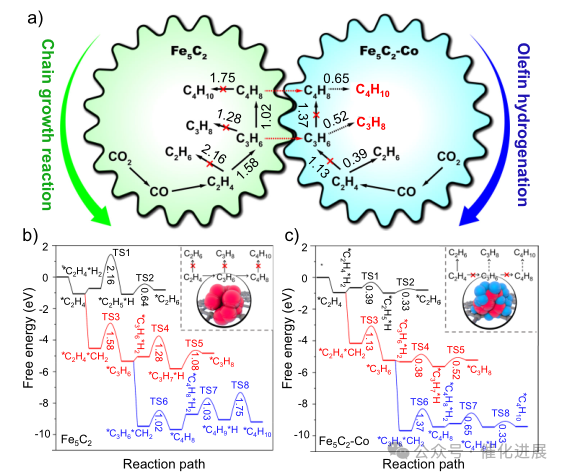
Fig. 5 Fischer-Tropsch reaction route of CO2 hydrogenation to LPG designed
In order to clearly explore the synergistic effect of double active sites in the reaction process, the author calculated the chain growth at Fe5C2 and Fe5C2- co sites and the hydrogenation reaction of olefins from ethylene to butane by DFT (Figure 5, supplementary Figures 35-37). At Fe5C2 site, olefins are more prone to C-C coupling reaction to realize carbon chain growth because of their lower free energy barrier, rather than secondary hydrogenation reaction.
Therefore, more long-chain olefins can be obtained. At the site of Fe5C2Co, the hydrogenation of olefins to alkanes is easier than chain growth reaction, so ethane is produced more than propane and butane. However, once the products of propylene and butene diffuse from the site of Fe5C2 to the site of Fe5C2- Co, propylene and butene are easily hydrogenated to propane and butane due to the low energy barriers (0.52 eV and 0.65 eV), which leads to high selectivity of LPG products.
To sum up, the author successfully prepared a graphene-regulated Fe-Co bimetallic catalyst with uniform active sites or dispersed dual active sites, and applied it to the hydrogenation of CO2 to selectively produce light olefins or LPG without any post-treatment. The GO/K-Fe-Co catalyst has a uniform distribution and a small particle size, with C2 = -C4 = selectivity as high as 50.1% and CO2 conversion of 55.4%. On the other hand, the GO-Fe/K-CO catalyst separated by graphene fence has a selectivity of 43.6% for LPG (propane and butane), 2.2% for CO and 46% for carbon dioxide conversion without any zeolite. The reaction result corresponds to the highest sty (151.0 g kg cat1h1) of LPG ever reported. The results of characterization and theoretical calculation show that the double active sites (iron carbide and metal cobalt) play their respective functions, meet the requirements of suitable carbon chain growth and olefin secondary hydrogenation, and selectively improve the selectivity of LPG. Finally, this work not only provides a multi-active site catalyst with unique spatial distribution for selective CO2 hydrogenation, but also provides a basic understanding of the role of graphene fence in selective hydrogenation.
Source:
1. LeiGao 1,5, TulaiSun 2,5, XuliChen 1,5, ZhilongYang 1, MengfanLi 1, WenchuanLai1, Wenhua Zhang3, Quan Yuan 1 & Hongwen Huang1,4. Nature Communications| (2024) 15:508
2. https://mp.weixin.qq.com/s/R1XjFVRQplj0WiRc59v-ug
