In2O3 @ Ni Selective Photocatalytic Conversion of CO2 to Methane

Photothermal CO2 methanation is an important research approach to achieve the goal of zero net emission, and plays a key role in formulating carbon neutralization, hydrogen utilization, carbon cycle and chemical energy storage strategies. Ni catalyst has attracted extensive attention in CO2 hydrogenation due to its excellent catalytic activity and stability. However, it is still challenging to identify the specific active centers of various products in Ni-based catalysts because the coordination structure of Ni species is complicated. These catalysts have irregular morphology, different particle sizes, uneven aggregates and unsaturated atoms at edges, steps and corners, which provide multiple active sites, leading to competitive intermediate reactions and the formation of multiple products, reducing the rate and selectivity of CO2 hydrogenation. In order to overcome these challenges, it is very important to ensure the accuracy and consistency of reaction sites.
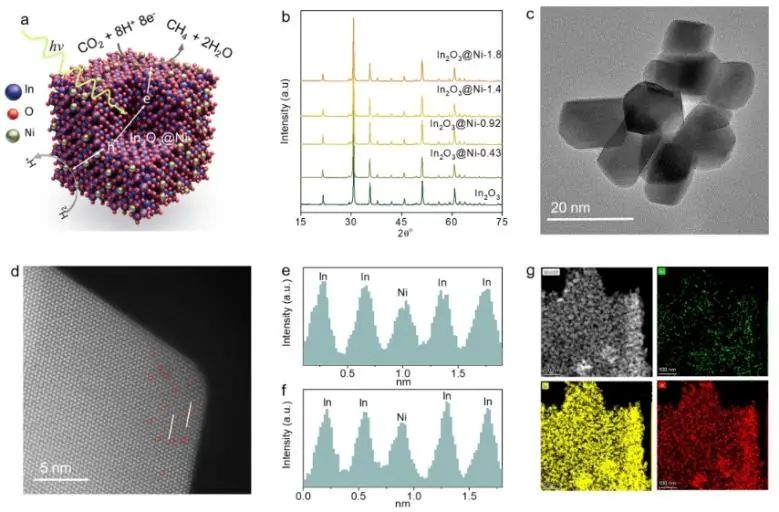
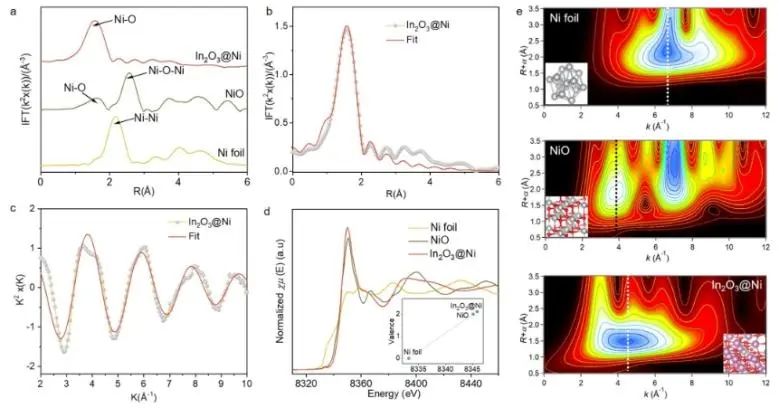
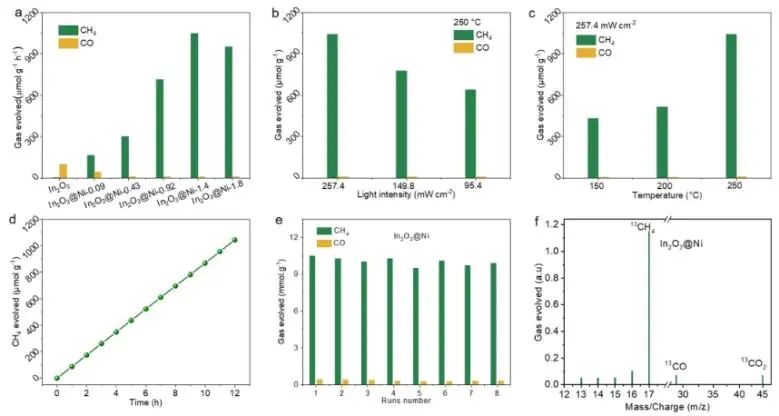
Monoatomic catalysts with clear active centers provide an ideal platform for exploring accurate structure-activity relationship at atomic scale. Recently, Zhang Huabin, Jorge Gascon from King Abdullah University of Science and Technology and Li Qiaohong from Fujian Institute of Structure, China Academy of Sciences have anchored uniformly dispersed monoatomic Ni centers on the surface of In2O3 (In2O3 @Ni). This catalyst has a clear coordination structure and is used to catalyze CO2 hydrogenation to CH4 with high selectivity. The experimental results show that the catalyst In2O3@Ni has excellent catalytic activity. Under simulated photothermal conditions, the selectivity of CO2 hydrogenation to CH4 is as high as 99%, and the hydrogenation rate reaches 1043 μ mol g1h1. The production rate of CH4 is 200 times that of photocatalysis and 6 times that of thermal catalysis. In addition, after 80 hours of continuous photothermal catalytic reaction, the catalyst still showed good activity, and there were no clusters or particles on the surface of the sample caused by Ni aggregation after the reaction.
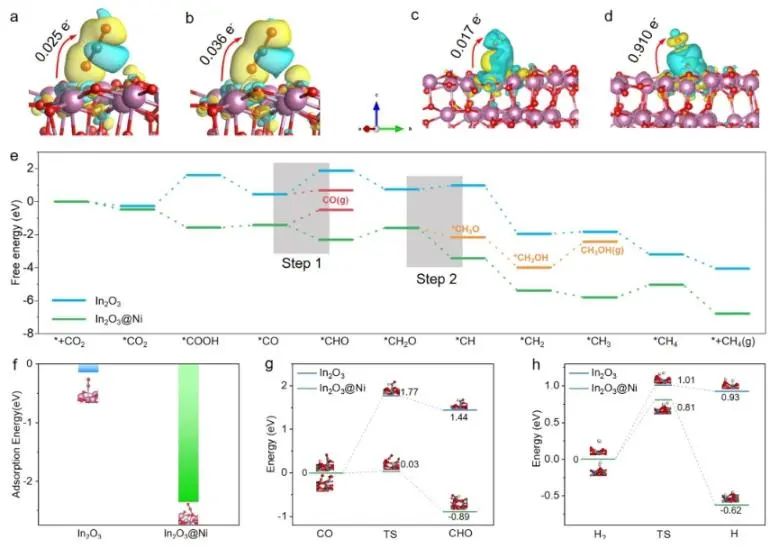
The calculation results of CO-TPD and density functional theory (DFT) show that the introduction of Ni atoms into the In2O3@Ni catalyst is helpful for the transfer of photogenerated electrons to CO2. At the same time, the introduction of Ni enhances the adsorption of CO* and reduces the formation energy barrier of COH* intermediates. In addition, In2O3@ Ni catalyst can accelerate the dissociation of H2 to generate H, and provide more protons for CO2 hydrogenation, thus improving the selectivity of CO2-CH4.
Generally speaking, this work proves that monoatomic catalysts are helpful to reveal the structure-activity relationship, and the strategies reported in this study can guide the design and development of new monoatomic catalysts and apply them to the fields of green energy and environment.
Reference
[1]Isolated Ni atoms enable near-unity CH4 selectivity for photothermal CO2 hydrogenation. Journal of the American Chemical Society, 2024. DOI: 10.1021/jacs.4c05873
Source:https://mp.weixin.qq.com/s/2IBbQQ2CdSxUW2J8f98sDw
