Analysis of the interface competition between hydrogen evolution and carbon dioxide reduction in photothermal catalysis by the regulation of edge active sites
Using solar energy to convert CO2 and H2O into high value-added products is an effective way to solve the problems of energy shortage, climate change and environmental pollution. Among them, solar-driven photothermal catalysis shows unique advantages and can realize efficient reaction under relatively mild operating conditions. However, due to the chemical inertness of CO2 and H2O molecules, the unfavorable energy absorption process in thermodynamics and the complex proton-coupled multi-electron transfer, the efficient conversion of CO2 and H2O without sacrificial agent still faces great challenges. Especially, the competitive HER in proton-coupled multi-electron reduction process makes the selectivity of carbon products not ideal. As a common cocatalyst, noble metal nanoparticles can form active sites on semiconductors, and the special active sites at the edge of metal-semiconductor interface may play a key role in the competition between HER and CO2RR. In-depth study on the evolution and interaction of key intermediate groups on specific active sites and understanding the competition mechanism between HER and CO2RR at the atomic level are very important for rational design of active sites to improve the selectivity of CO2RR.
1 noble metal supported Al-doped SrTiO3 catalyst
Al-doped SrTiO3 (ASTO) was synthesized by molten salt-mediated method, and a series of noble metal-semiconductor interfaces (M/ASTO, M=Ru, Rh, Pd, Ag, Pt and Au) were constructed by loading different noble metal nanoparticles by photo-deposition. Among them, Ru, Rh, Pd and Pt nanoparticles have relatively small particle sizes, all less than 10 nm, while Ag and Au form some nanoparticles with diameters over 30nm. The XPS fine spectrum of precious metals shows that Ru, Pd and Pt exist only in oxidized form. Rh and Ag are mainly in oxidation state, accompanied by a small amount of metal state; While Au remains in a pure metallic state. It shows that the smaller the size of metal nanoparticles, the higher the proportion of surface atoms and atoms strongly coupled with semiconductor carriers, which leads to the enhancement of chemical reaction activity and electron transfer. The edge sites of metal-semiconductor interface with high abundance on small-sized nanoparticles are expected to play a more critical role in the catalytic process.
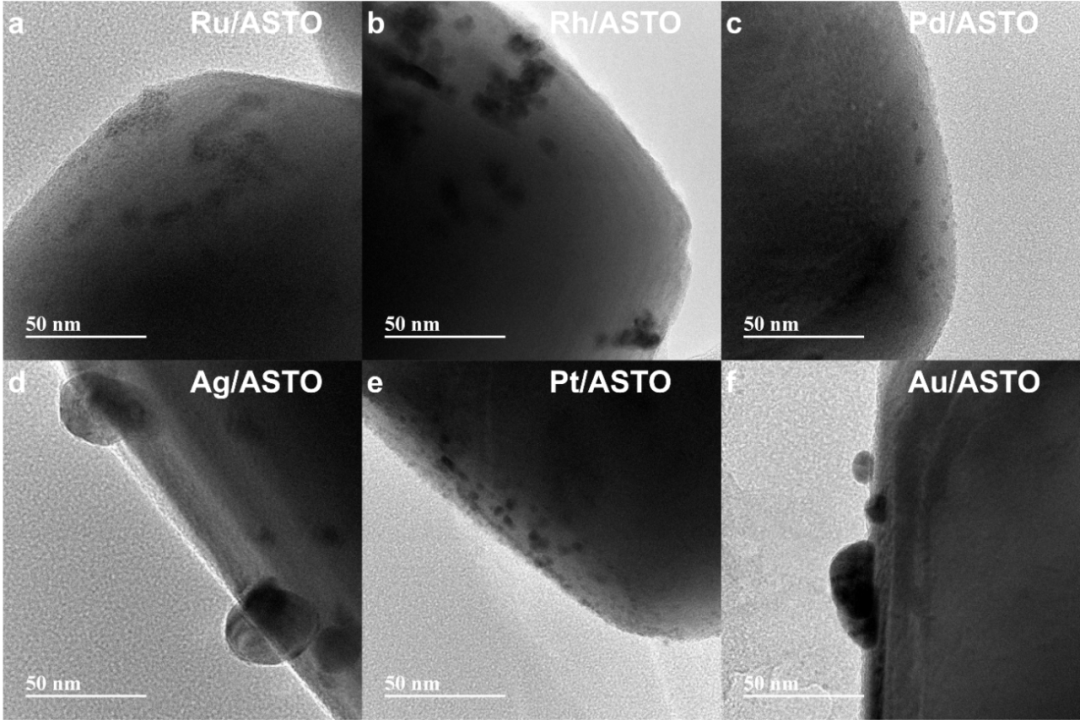
Fig. 1 TEM images of (a) Ru/ASTO, (b) Rh/ASTO, (c) Pd/ASTO, (d) Ag/ASTO, (e) Pt/ASTO and (f) Au/ASTO.
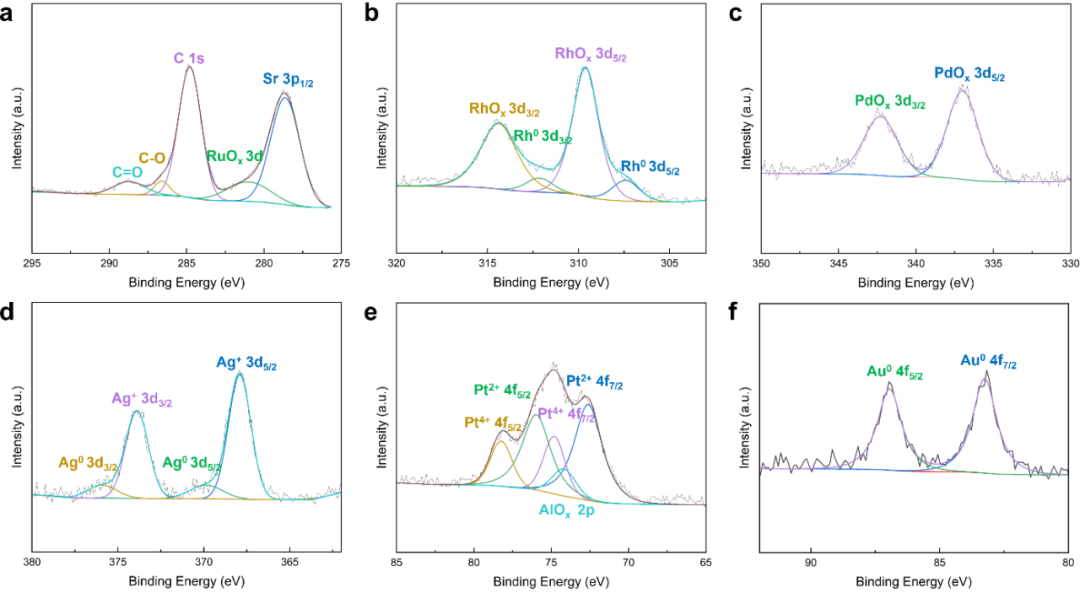
Fig. 2 XPS fine spectra of (a) Ru 3d, (b) Rh 3d, (c) Pd 3d, (d) Ag 3d, (e) Pt 4f and (f) Au 4f of M/ASTOs.
2 Effect of CO2 on hydrogen evolution in photothermal catalysis
The gas-liquid interface reaction system is used to make CO2 and H2O fully contact the active sites at the same time, and the local thermal effect is used to promote the reaction kinetics. In the continuous photothermal catalysis experiment, different noble metal nanoparticles promoted the formation of H2 to varying degrees. However, it is disappointing that the production rate of CO and CH4 is 2-4 orders of magnitude lower than that of hydrogen production, and HER is dominant in the competition. Comparing the hydrogen production rate with or without CO2, we found that the effect of CO2 on hydrogen evolution performance on different noble metal nanoparticles was significantly different. When CO2 was introduced, the HER activity of Rh/ASTO was most significantly inhibited. The hydrogen production rates of Pt/ASTO and Au/ASTO were less inhibited. The hydrogen production rates of Ru/ASTO and Pd/ASTO were increased due to the introduction of CO2. The hydrogen evolution activity of Ag/ASTO is the weakest, and its hydrogen production performance is also the least sensitive to CO2 atmosphere.
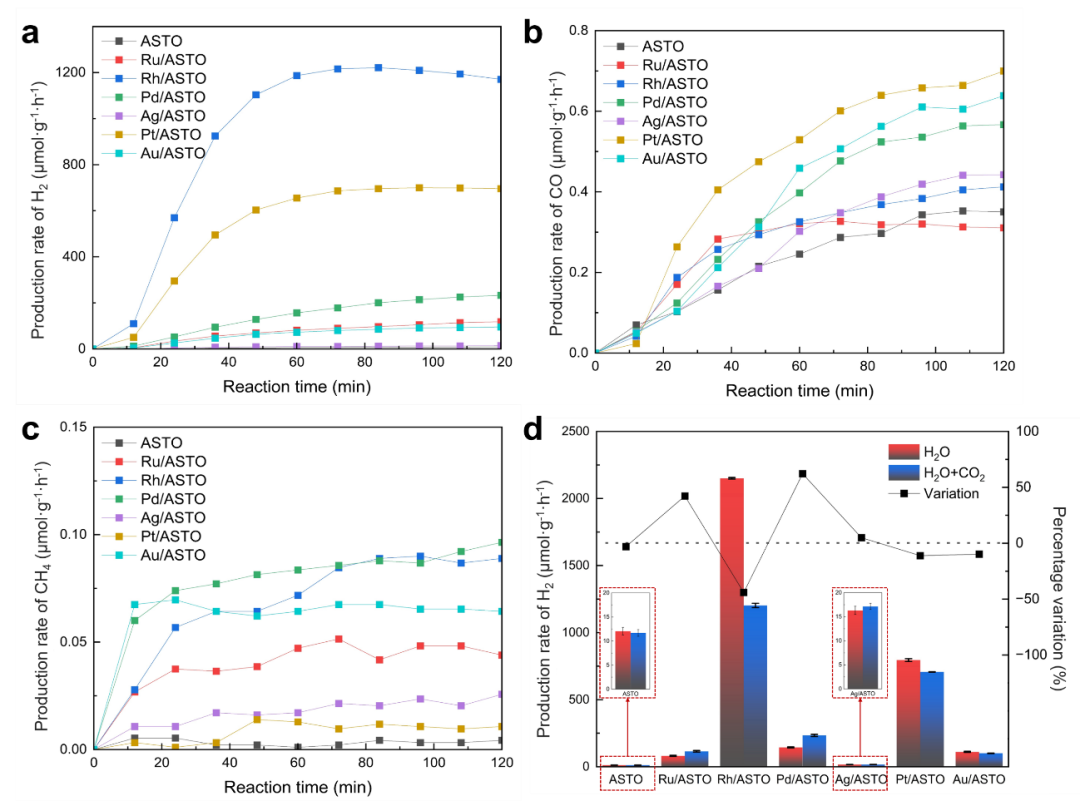
Fig. 3 Changes of the formation rates of (a) H2, (b) CO and (c) CH4 with time during the photothermal catalytic conversion of CO2 and H2O on ASTO and M/ASTO; (d) Hydrogen production rate and percentage change rate of ASTO and M/ASTOs with or without CO2.
3 Interaction of key intermediate groups
In-situ diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS) was used to monitor the dynamic evolution of intermediate groups in the reaction. Compared with ASTO, the intensity of CO2-related characteristic peaks on M/ASTO is obviously enhanced, which indicates that noble metal nanoparticles effectively promote the adsorption and activation of CO2. After the introduction of CO2, several new characteristic peaks of intermediate groups appeared in the range of 1100-2300 cm−1. Among them, the proportion of bidentate carbonate (b-CO32-) in Rh/ASTO, Pt/ASTO, Ag/ASTO and Au/ASTO is higher than that in Ru/ASTO and Pd/ASTO. According to the configuration of b-CO32- adsorption, it can be inferred that the edge of metal-semiconductor interface provides a large number of sites for b-CO32- adsorption. The edge atoms of noble metal nanoparticles are occupied, which leads to the decrease of HER sites. In addition to the blocking effect of b-CO32- on the interface edge sites, b-CO32- may also affect the hydrogen evolution barrier of adjacent sites. DFT calculation results show that the existence of b-CO32- at the metal-semiconductor interface increases the hydrogen evolution barrier of Rh/ASTO and Pt/ASTO, but decreases the hydrogen evolution barrier of Ru/ASTO and Pd/ASTO. Among these catalysts, Rh/ASTO shows a high proportion of b-CO32- and a small change in hydrogen evolution energy barrier. In view of the fact that CO2 significantly inhibited the hydrogen evolution activity of Rh/ASTO, the site blocking effect of b-CO32- is the key factor that dominates the competition between HER and CO2RR on the surface of Rh/ASTO.
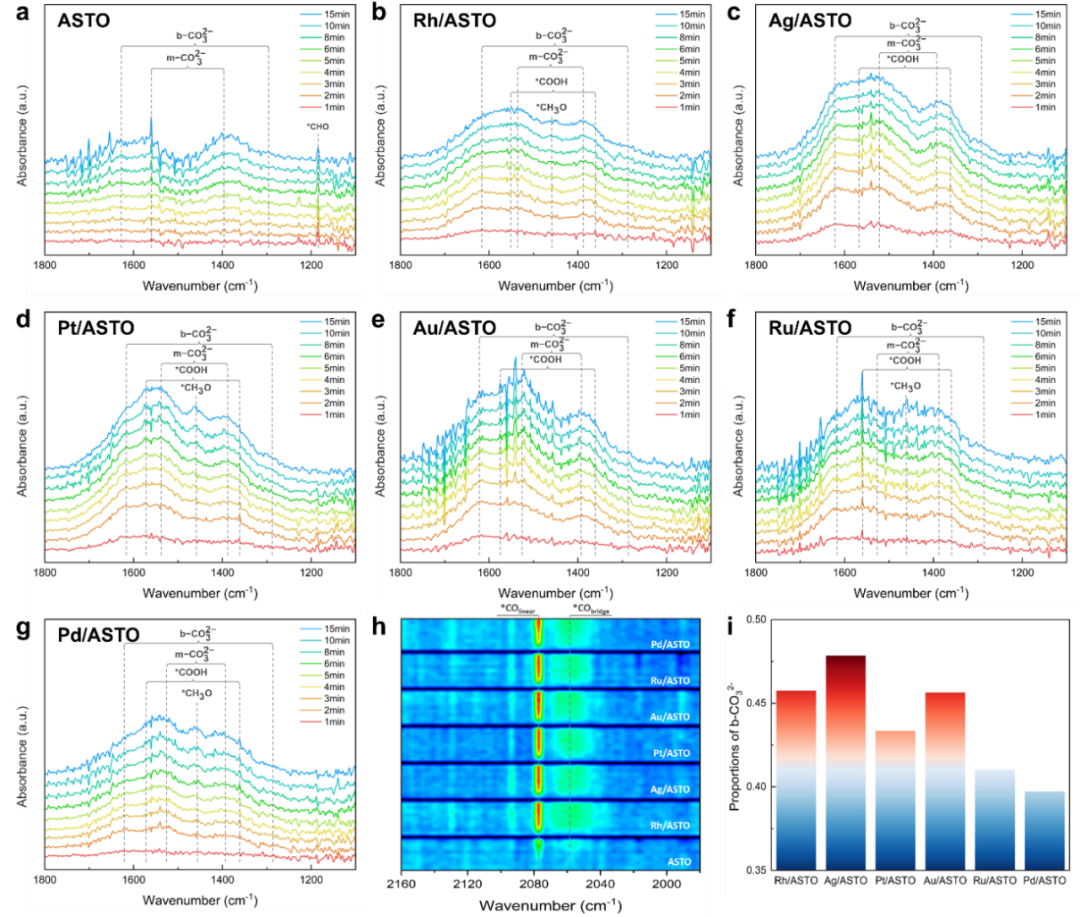
Fig. 4 (a–g) in-situ time-resolved DRIFTS and (h) in-situ time-resolved DRIFTS of ASTO and M/ASTO irradiated (< 800 nm) under the coexistence of CO2 and H2O; (I) the proportion of b-CO32- on m/ASTOs.
4 Blocking effect of b-CO32- at the edge of metal-semiconductor interface
In order to verify the site blocking effect of b-CO32- on hydrogen evolution reaction, we prepared ASTO with different Rh particle sizes by changing Rh loading or preparation method. The experimental results show that the change of hydrogen production rate caused by CO2 is closely related to the particle size of Rh: the larger the size of Rh nanoparticles, the weaker the inhibition effect of CO2 on hydrogen evolution. In order to quantify the relationship between particle size and active site ratio, a simplified geometric model was established. When the site blocking effect of b-CO32- is the dominant factor in the competition between HER and CO2RR, the decay rate of hydrogen production rate can be expressed as the ratio of the number of atoms at the interface edge to the total number of atoms on the surface. According to the change of hydrogen production rate measured by experiments, the equivalent particle size of Rh particles can be deduced. When Rh loading is low, particles with sizes matching the calculated results can be observed in TEM images. However, the deterioration of hydrogen evolution performance of large-size Rh nanoparticles makes the deviation between equivalent size and actual particle size gradually increase under high load.
The protonation process of CO2RR requires moderate *H coverage and smooth proton transfer. With the decrease of Rh particle size, the hydrogen production rate per unit mass of Rh shows a monotonic upward trend, but too small or too large Rh particles are not conducive to CH4 production. When the particle size of Rh is too small, the proportion of edge sites at the metal-semiconductor interface increases, which leads to excessive adsorption of carbonate species, which will competitively consume the active sites needed for *H adsorption, thus reducing the coverage of *H and reducing the protonation efficiency. The balance between *H coverage and CO2 activation can be achieved by Rh nanoparticles with moderate size, and 0.5-Rh/ASTO shows the highest CH4 generation rate, which is about 38 times that of ASTO. However, when the particle size exceeds the optimal range, the proton migration efficiency will decrease due to the extension of the average migration path, and the methane production will drop sharply.
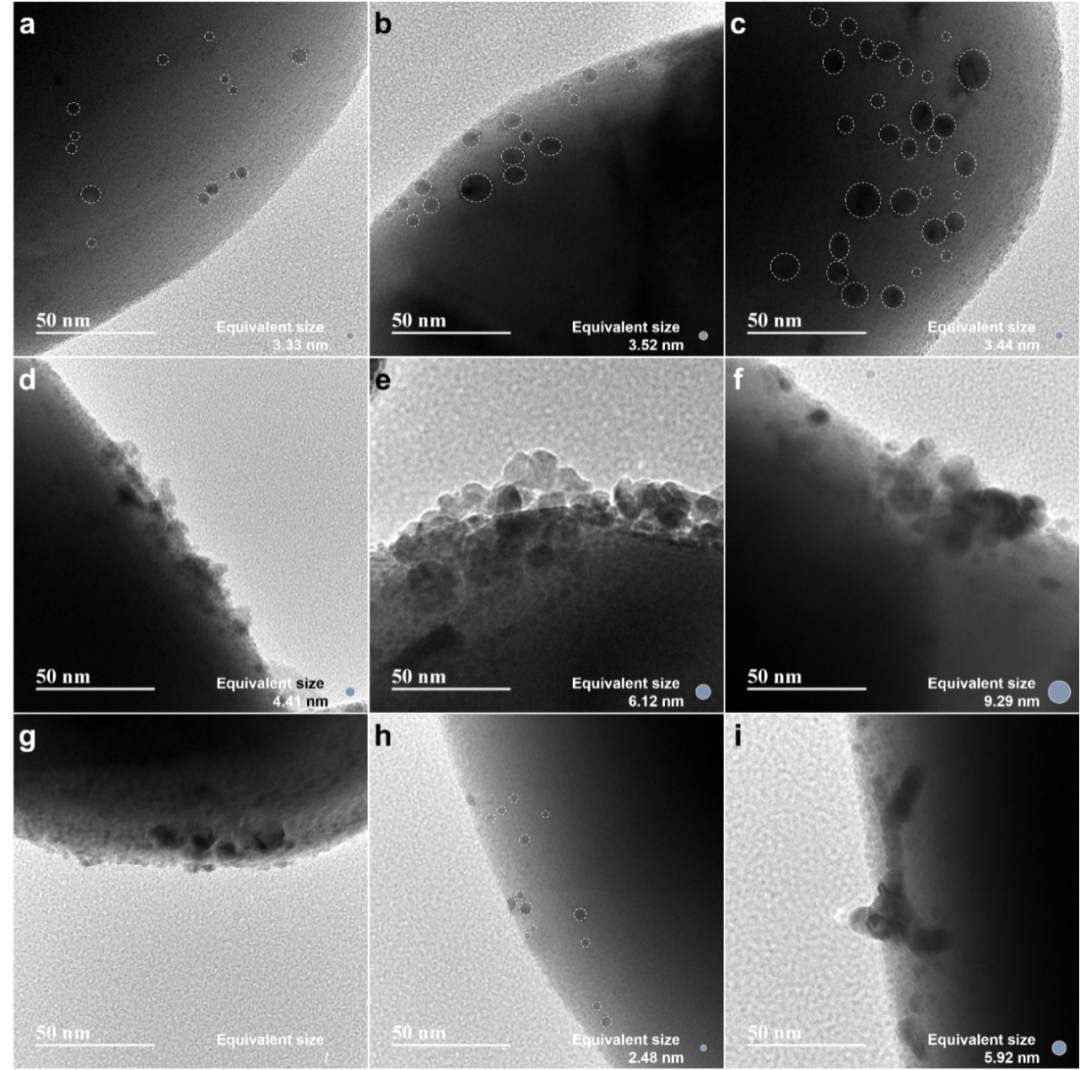
Fig. 5 (a) 0.05-Rh/ASTO, (b) 0.1-Rh/ASTO, (c) 0.2-Rh/ASTO, (d) 0.5-Rh/ASTO, (e) 1.0-Rh/ASTO, and (f) 2.0-Rh/ASTO.
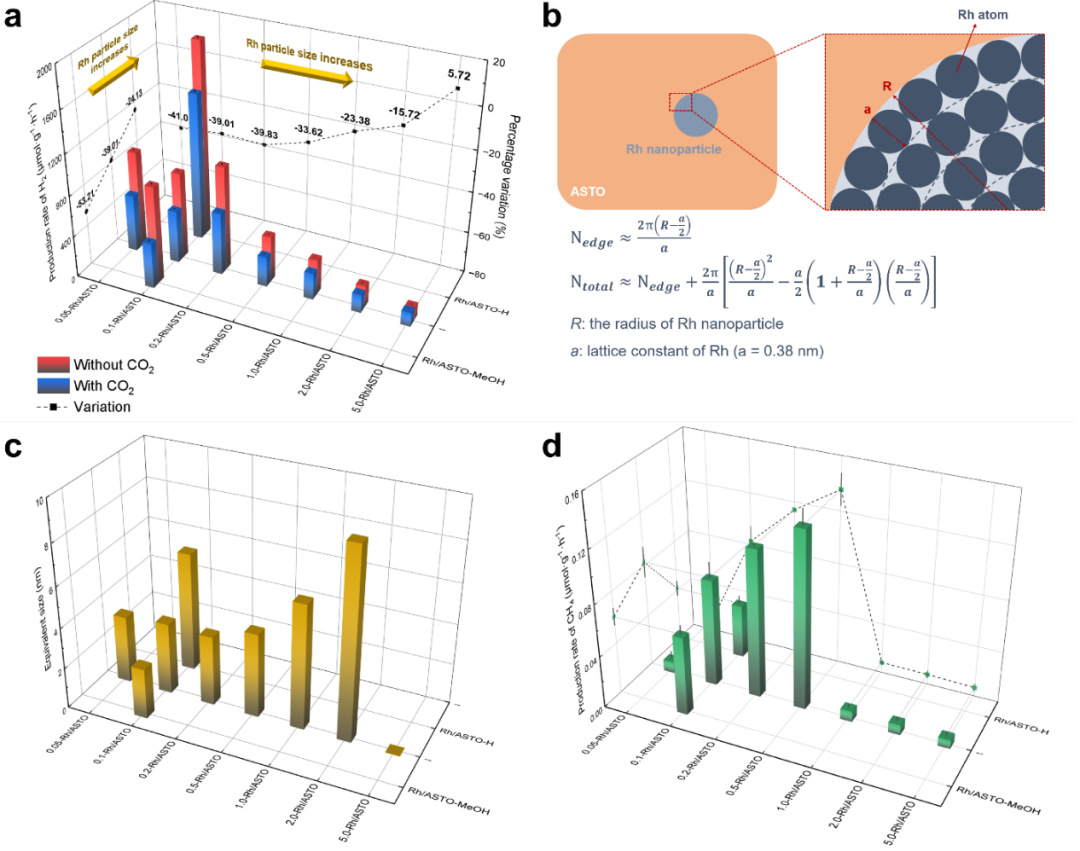
Fig. 6 (a) x-Rh/ASTO (x = 0.05, 0.1, 0.2, 0.5, 1.0, 2.0 and 5.0), hydrogen production rates and percentage changes of Rh/ASTO-MeOH and Rh/ASTO-H with or without CO2; (b) The simplified geometric model of RH particles and the estimation formula of the number of atoms at the edge of the metal-semiconductor interface and the total number of atoms on the surface (Nedge and Ntotal); (c) Equivalent size of RH particles; (d) x-Rh/ASTO (x = 0.05, 0.1, 0.2, 0.5, 1.0, 2.0 and 5.0), and CH4 production rates of Rh/ASTO-MeOH and RH/ASTO-H.
Conclusion and prospect
Combined with in-situ DRIFTS and DFT calculations, the competition mechanism between HER and CO2RR at the metal-semiconductor interface was preliminarily revealed through the regulation of edge active sites, and the process of methane generation from CO2RR was promoted by balancing hydrogen evolution and CO2 protonation. However, the formation of carbon products is still limited by the toxic effect of *CO and the dynamic challenge of proton-coupled multi-electron transfer. More comprehensive and systematic research is needed to clarify the potential competition mechanism between HER and CO2RR, and the competition of HER can be suppressed through reasonable design of catalytic materials. In order to realize the efficient conversion of CO2 and H2O, the following key factors should be considered in the design of catalytic active sites: (1) constructing multiple active sites to promote the adsorption and activation of CO2 and H2O respectively; (2) Maintain moderate adsorption strength for key intermediates to prevent premature desorption or catalyst poisoning; (3) Strategically design the spatial arrangement of active sites to optimize proton transfer in the reaction process.
Source:https://www.whxb.pku.edu.cn/CN/10.1016/j.actphy.2025.100099
