Tianjin University Researchers Develop Biomimetic Catalyst for Efficient Recycling of Polyester Plastics
Overview
The global plastic waste challenge demands scalable and energy-efficient solutions. In a study recently published in Chemical Science, a team from Tianjin University developed a biomimetic Zn–Salen molecular catalyst capable of depolymerizing polyethylene terephthalate (PET) under mild conditions. Inspired by the mechanisms of PET-hydrolyzing enzymes and Salen-type complexes, the catalyst enables efficient conversion of post-consumer PET waste into valuable monomers.
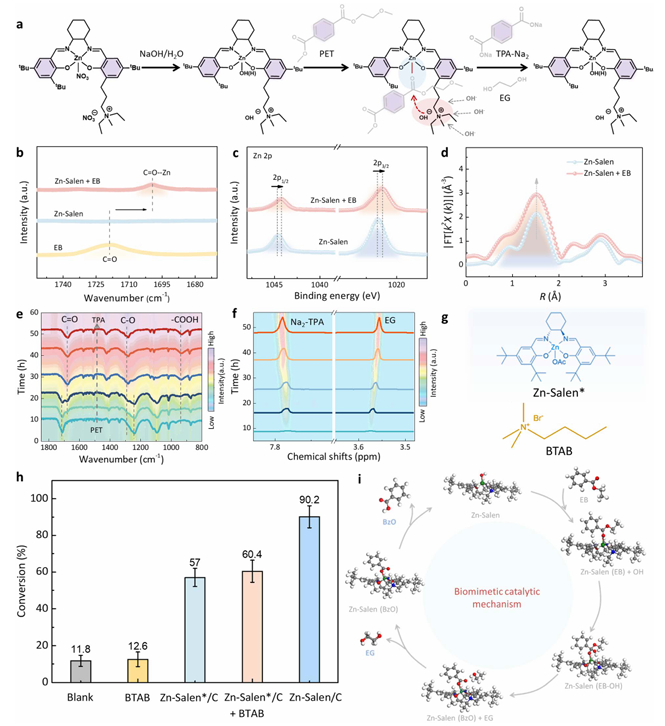
The Zn–Salen catalyst utilizes a synergistic proximity effect between Zn metal centers and quaternary ammonium groups to enhance substrate adsorption, activation, and nucleophilic cleavage. It exhibits strong tolerance to complex waste streams—including mixed plastics and biodegradable PLA—and shows significant advantages in both reactivity and sustainability compared to traditional and enzymatic recycling methods.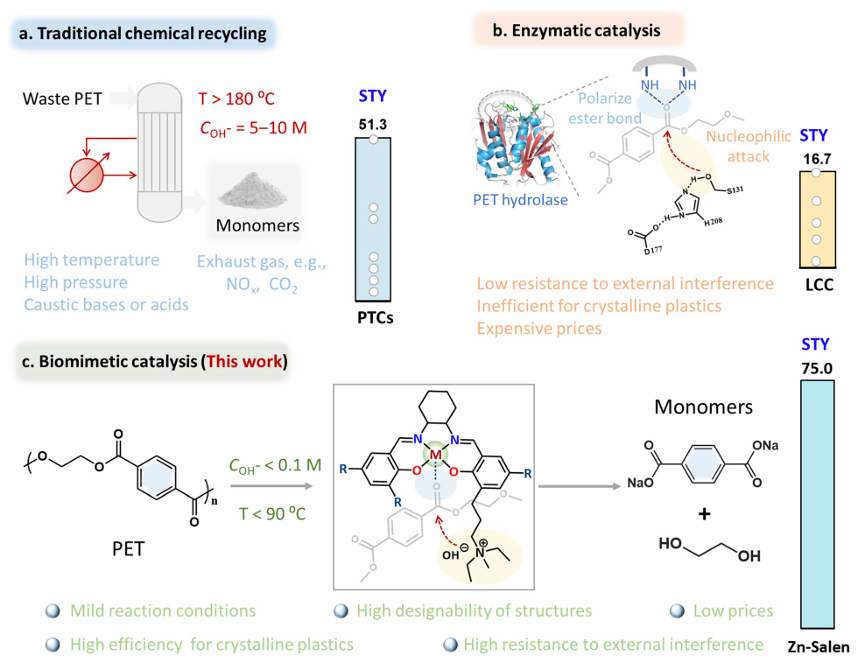
Catalyst Design and Performance
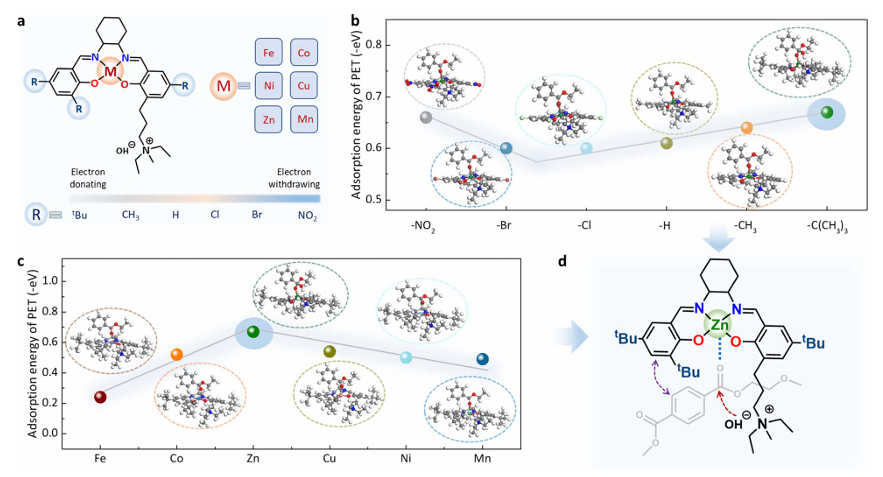
Through DFT-based screening, the team identified a Robson-type Zn–Salen complex (with tert-butyl substituents) as the most effective candidate for PET adsorption. To facilitate recovery and reuse, the catalyst was immobilized on carbon black nanoparticles, forming Zn–Salen/C.
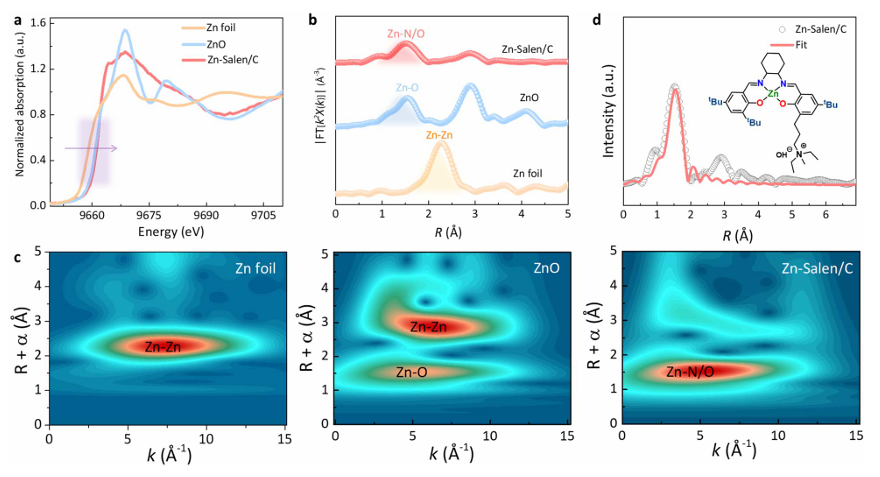
Advanced structural characterization confirmed that the catalyst retained its molecular structure upon loading. Spectroscopic analyses showed that the Zn sites remained in a high oxidation state and maintained their coordination environment, key for activating PET ester groups.
The catalyst achieved a space–time yield of 75 g TPA·L⁻¹·h⁻¹ at 90 °C in dilute alkaline solution (0.1 M), far outperforming existing methods. It also demonstrated:
>70% conversion efficiency for complex PET-containing waste (textiles, plastic mixtures)
68–88% conversion of biodegradable PLA and post-consumer plastic products
High selectivity and stability under realistic conditions
Mechanistic Insights
A biomimetic catalytic cycle was proposed based on enzymatic PET hydrolysis:
Adsorption of PET onto the catalyst surface;
Zn–O=C coordination to activate the ester bond;
Nucleophilic attack by hydroxyl groups enhanced by quaternary ammonium proximity;
C–O bond cleavage and desorption of monomeric products.
Evidence from DRIFTS, FT-IR, ¹H NMR, and control experiments validated this mechanism. DFT calculations confirmed that the Zn site stably binds the PET carbonyl group, while quaternary ammonium units enhance nucleophilic reactivity and facilitate active site regeneration. The overall energy profile supports the reaction’s thermodynamic feasibility under mild conditions, demonstrating that the system mimics PET hydrolases through a cooperative proximity effect between Zn and quaternary ammonium units.
Conclusion
This work demonstrates a scalable, tunable, and stable catalytic platform for sustainable plastic recycling. The Zn–Salen/C catalyst combines high performance with operational simplicity, broad substrate compatibility, and significant commercial promise.
Looking ahead, the tunability of Zn–Salen complexes offers opportunities for further optimization toward enhanced durability and selectivity—paving the way for next-generation biomimetic recycling technologies.