Researchers at Huazhong University of Science and Technology Reveal Key Mechanisms in Low-Temperature Dehydrochlorination and Pyrolysis of PVC
A research team from the State Key Laboratory of Coal Combustion, Huazhong University of Science and Technology (HUST) has made significant progress in understanding the low-temperature dehydrochlorination and pyrolysis behavior of polyvinyl chloride (PVC), one of the world’s most widely used plastics.
Their findings, published in Waste Disposal & Sustainable Energy under the title “Dehydrochlorination of PVC at low temperatures and the pyrolysis behavior of dechlorinated PVC,” provide critical insights into how PVC decomposes and transforms at low temperatures—offering a theoretical foundation for environmentally friendly PVC recycling and plastic waste management.
PVC, accounting for approximately 13% of global plastic production, poses serious challenges for thermal recycling due to its high chlorine content and toxic byproducts. To address this, the HUST team—led by Professor Jie Yu, with Jiayou Sun and Tianyang Ding as co-first authors—conducted systematic dehydrochlorination experiments in the 170–220 °C range using fixed-bed reactors.
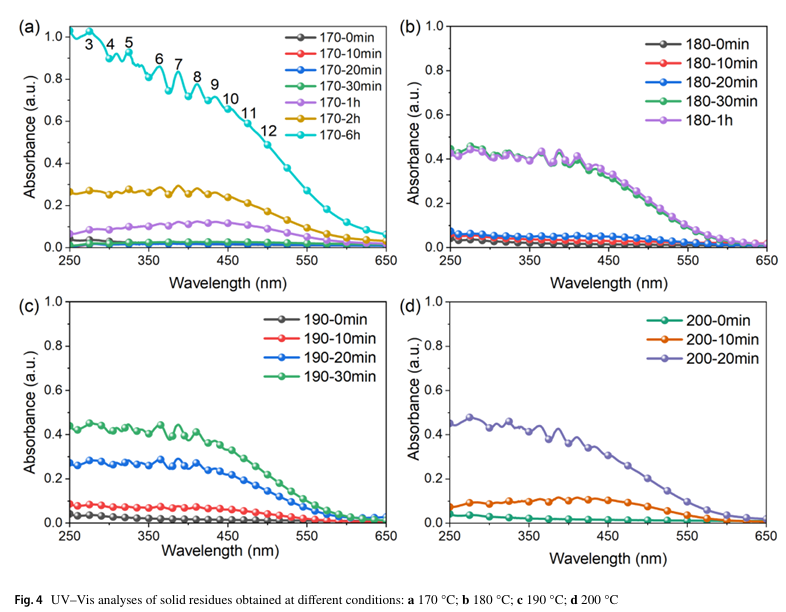
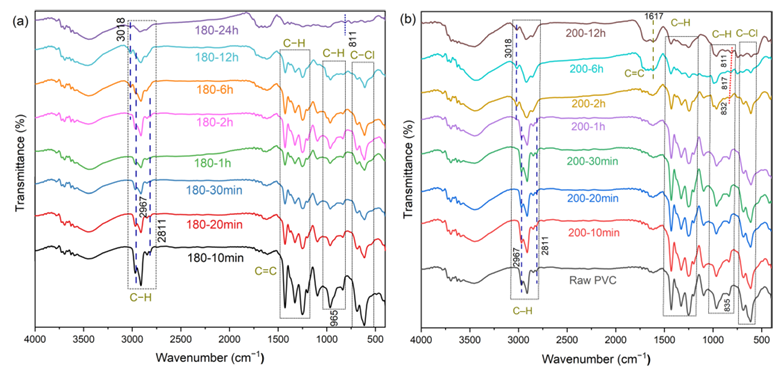
They characterized the intermediate structures and reaction pathways using UV–Vis, Raman, and FTIR spectroscopies, combined with pyrolysis-gas chromatography/mass spectrometry (Py-GC/MS) to identify decomposition products.
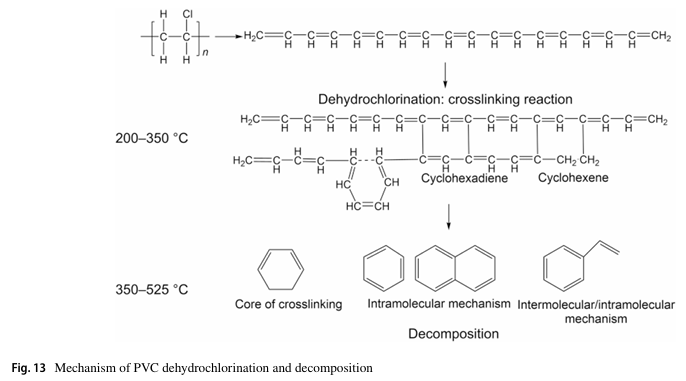
The results revealed that PVC undergoes a two-stage decomposition process. In the initial stage, soluble intermediates rich in conjugated polyene sequences are formed without crosslinking, accompanied by the release of HCl. As the reaction progresses, these intermediates transform into highly crosslinked, insoluble black solids, dominated by cyclohexene and cyclohexadiene structures. Upon further pyrolysis, the dehydrochlorinated residues yield a wide range of aromatic compounds, particularly benzene and naphthalene, while crosslinking reactions suppress benzene formation and enhance alkyl-aromatic production.
Importantly, this study clarifies the molecular evolution of PVC during low-temperature treatment, revealing that crosslinking reactions and conjugated polyene growth are central to the transformation from initial intermediates to stable carbonaceous residues. The proposed mechanism bridges the gap between PVC dehydrochlorination and pyrolysis, paving the way for controlled thermal recycling processes that minimize chlorine emissions and produce valuable hydrocarbons.
“By precisely tracking the chemical transitions in dechlorinated intermediates, we’ve identified how low-temperature conditions alter the molecular architecture of PVC,” said Professor Jie Yu.
“This understanding will guide the development of cleaner, more efficient methods for PVC waste recycling and resource recovery.”
This research was supported by the Special Funds for Guiding Local Scientific and Technological Development by the Central Government of China , the National Natural Science Foundation of China , and the Natural Science Foundation of Wuhan .