ECUST Scientists Engineer Oxygen-Retaining Oxide-Derived Copper for Efficient CO₂ Reduction to C₂+ Products
Researchers at East China University of Science and Technology have unveiled a new strategy to boost the production of multi-carbon products from electrochemical CO₂ reduction, by deliberately retaining a controlled amount of oxygen inside oxide-derived copper (OD-Cu) catalysts. The work, recently published in Angewandte Chemie, offers a fresh design rule for copper-based catalysts that convert waste CO₂ and intermittent renewable electricity into higher-value chemicals and fuels.
Electrochemical CO₂ reduction (CO₂RR) on copper is one of the most promising routes to store renewable power in the form of carbon-based products such as ethylene, ethanol and other C₂+ species. Among the many copper catalysts explored, oxide-derived copper has drawn particular attention because its nanostructured surface can be tuned to favor multi-carbon products. However, what the authors call the “residual oxygen problem” has remained poorly understood: during the complex reduction and reconstruction of copper oxides, part of the original oxygen remains in the catalyst, and how to control this oxygen—and whether it is beneficial or harmful—has been an open question.
In this study, the team used CuO nanosheets as a well-defined model precatalyst to isolate and probe the role of residual oxygen. By precisely adjusting the nanosheet thickness, they were able to regulate how much oxygen is retained in the resulting OD-Cu and correlate this with structure and performance.
The results are striking. When the CuO nanosheets are thinned down to about 1.6 nm, the reconstructed OD-Cu shows a greatly enhanced ability to “hold onto” oxygen. Under industrially relevant current densities of 300–700 mA cm⁻², this ultrathin OD-Cu catalyst achieves around 80% Faradaic efficiency for C₂+ products, with a peak value of 84.6% at 700 mA cm⁻². At that point, ethylene accounts for 58.4% of the current, ethanol 19.8%, acetic acid 4.0% and n-propanol 2.5%, while hydrogen evolution is effectively suppressed. The catalyst also maintains high selectivity and activity over extended operation, remaining stable for at least 8 hours at 400 mA cm⁻² with ethylene efficiency close to 50% and H₂ selectivity below 10%.

To understand why residual oxygen is so beneficial, the researchers combined long-timescale molecular dynamics simulations with a suite of advanced structural and electrochemical characterizations. On the theory side, they modelled CuO-derived Cu–CuO structures with different degrees of deoxygenation. As more oxygen layers are removed from the starting CuO (n = 1, 2, 4, 5), the remaining Cu–CuO framework becomes more stable and the deeper oxygen atoms become increasingly difficult to dissolve or diffuse out. Calculated dissolution free energies (ΔGd) rise with each removed layer, indicating that the subsurface oxygen is effectively “locked in”.
This trapped oxygen has a direct catalytic consequence. On Cu–CuO surfaces with five oxygen layers removed, the free-energy barrier for hydrogenating adsorbed CO to the key intermediate CHO drops to 0.41 eV, compared to 0.67 eV on pure metallic Cu. In parallel, the energy barrier for coupling CO and CHO to form OC–CHO—a crucial C–C coupling step toward C₂+ products—is only 0.74 eV on Cu–CuO, significantly lower than the 1.2 eV barrier calculated for bare copper. In other words, the presence of residual oxygen in a stable Cu–CuO framework both facilitates CHO formation and accelerates C–C bond formation, thereby promoting multi-carbon product generation.
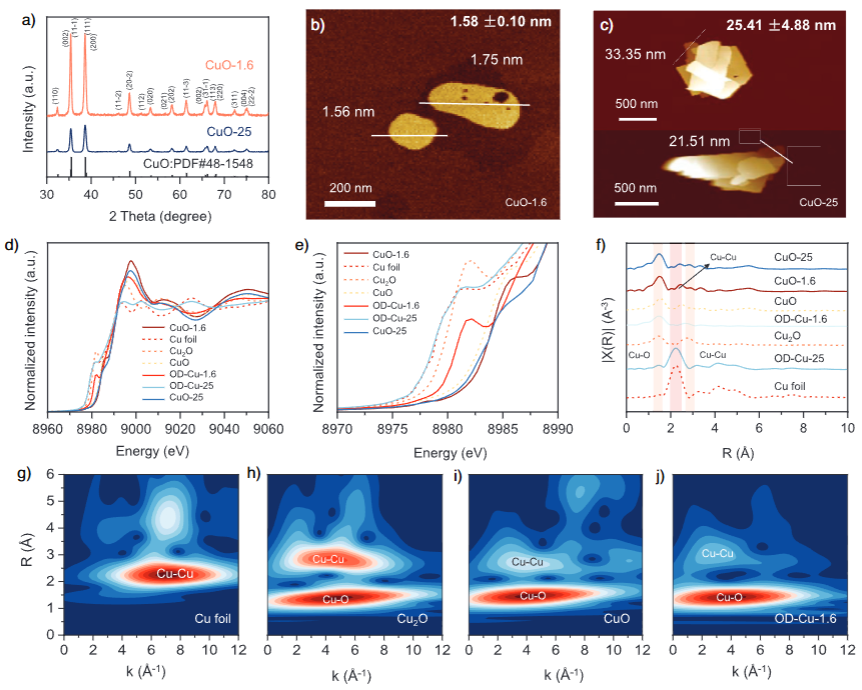
Experimentally, the team prepared CuO nanosheets with average thicknesses of about 1.6 nm (CuO-1.6) and 25.4 nm (CuO-25) as contrasting model precatalysts. X-ray diffraction confirmed that both materials share the same CuO crystal structure, ensuring that thickness is the main variable. Atomic force microscopy revealed uniform nanosheet morphology and validated the thickness control.
X-ray absorption spectroscopy at the Cu K-edge, before and after CO₂RR, provided direct evidence for residual oxygen and partially oxidized copper species in the ultrathin sample. XANES and EXAFS analyses, supported by Morlet wavelet transform, showed the coexistence of Cu–O and Cu–Cu coordination in OD-Cu-1.6 even under reducing conditions, a structural fingerprint of oxygen-containing Cu–CuO domains. In contrast, the thicker CuO-25-derived catalyst displayed a lower oxygen retention.
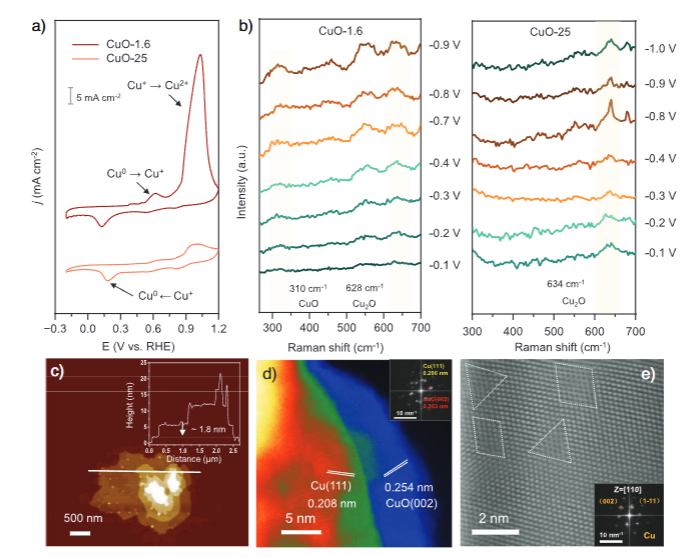
Electrochemical measurements further highlighted the different oxygen behaviors. Cyclic voltammetry after a strong cathodic treatment (700 mA cm⁻² for 1800 s in Ar) revealed that CuO-1.6 exhibits more pronounced oxidation peaks and is harder to fully reduce, consistent with a higher oxygen storage capacity. In situ Raman spectroscopy during CO₂RR showed that CuO-1.6 can retain CuO-related signals over a range of potentials, while the thicker sample is more readily reduced to metallic copper.
Microscopy offered a real-space picture of the reconstructed catalysts. AFM images of OD-Cu-1.6 indicated that the thinnest nanosheets after reconstruction remain close to the original 1.6 nm thickness, confirming the stability of the ultrathin layered structure. High-angle annular dark-field STEM images and their FFT patterns revealed stacked nanosheet architectures with lattice spacings corresponding to Cu(111) and CuO(002) planes, as well as abundant defects and nanoporosity. Aberration-corrected HAADF-STEM highlighted disordered regions rich in Cu atomic defects, providing sites that can interact strongly with reaction intermediates.
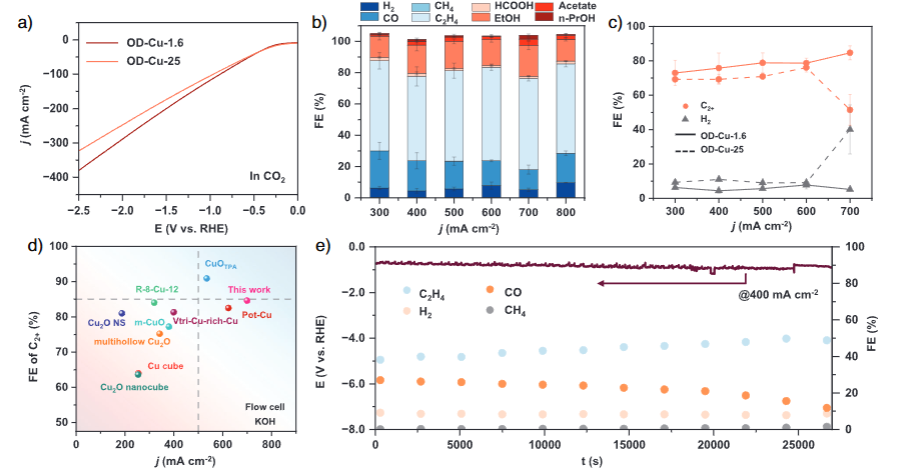
Functionally, these structural features translate into a catalyst surface that is both oxygen-rich and highly active. The stacked OD-Cu-1.6 nanosheets, with their pores and defects, favor OH⁻ adsorption and *CO enrichment, creating a local microenvironment conducive to C₂+ formation and unfavorable for the competing hydrogen evolution reaction. Linear sweep voltammetry confirmed that OD-Cu-1.6 delivers higher CO₂RR current densities than OD-Cu-25 under CO₂, underscoring its intrinsically higher activity.
Taken together, the study demonstrates that the thickness of CuO precatalysts provides a powerful handle to regulate residual oxygen in oxide-derived copper. Thinner CuO nanosheets lead to OD-Cu with stronger oxygen-holding capability, stable Cu–CuO domains, and metal centers that are easy to re-oxidize, all of which contribute to enhanced C₂+ selectivity at high current densities.
The authors conclude that residual oxygen is not merely a leftover from catalyst activation, but a key design parameter for OD-Cu in CO₂ electroreduction. By engineering oxygen retention and the resulting Cu–CuO interfaces, it is possible to unlock higher selectivity toward multi-carbon products under practical operating conditions. This oxygen-guided design concept may be extended to other copper-based and oxide-derived electrocatalysts, bringing efficient CO₂-to-fuels conversion a step closer to industrial reality.
