Cu-Zn-based alloy/oxide interfaces for enhanced electroreduction of CO2 to C2+ products
Recently, Professor Wang Zhongli of Tianjin University successfully synthesized Cu-Zn alloy /Cu-Zn aluminate composite catalyst by in-situ electroreduction of Cu-Zn oxide /Cu-Zn aluminate. Among them, alloys and oxides are closely stacked and alternately arranged, forming a large number of metal-oxide interfaces. Using the interface effect, the Faraday efficiency of the optimized Cu9Zn1/Cu0.8Zn0.2Al2O4 O4 catalyst for C2+ products reached 88.5% at-1.15 V vs Rhe and current density of 400 mA cm-2.
In-situ ATR-IRAS shows that aluminate oxides at the interface significantly enhance the adsorption and activation of CO2 and the dissociation of H2O, and accelerate the production of *CO intermediates on Cu-Zn alloy, while oxidized Cu enhances the adsorption of CO intermediates, and then Cu and Zn sites in Cu-Zn alloy synergistically promote C-C coupling. This work provides an effective strategy for constructing high-activity reaction interface of electrochemical CO2 reduction in industrial scale.
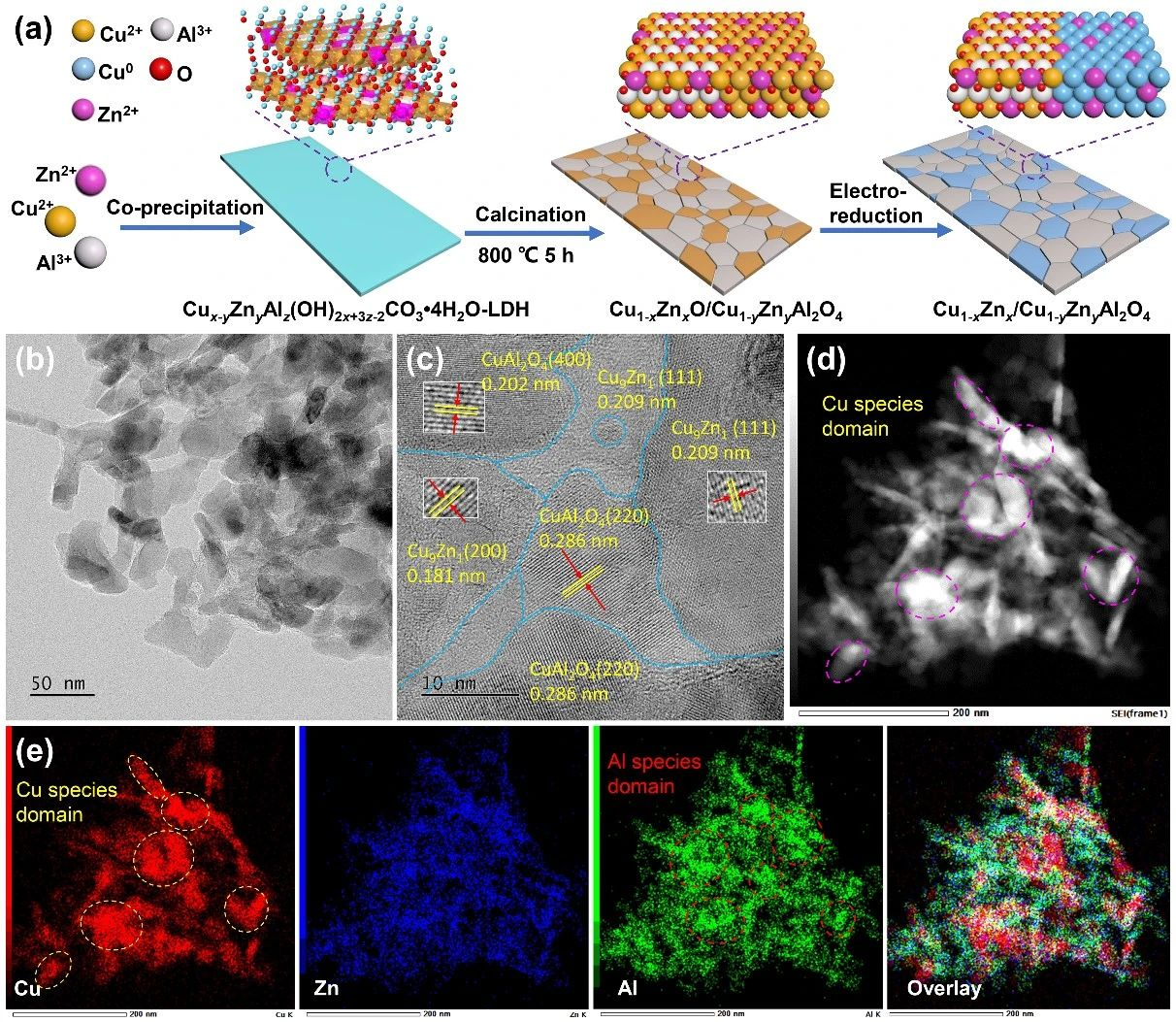
Fig. 1 synthesis and structural characterization of CuZn/CuZnAl2O4 catalyst (a) schematic synthesis diagram of CuZn/CuZnAl2O4 catalyst, (b)TEM image, (c)HR-TEM image, (d)HAADF-STEM image and (e)EDX element distribution map
In this work, CuZnAl- hydrotalcite (LDH) precursor was synthesized by coprecipitation method, and then the complex oxide rich in CuZnO-CuZn aluminate interface was obtained by heat treatment, and then it was electrochemically reduced in situ. Among them, CuZnO was reduced to CuZn alloy, while CuZn aluminate remained unchanged, and CuZn/CuZnAl2O4 catalyst was obtained. It is proved by transmission electron microscope, X-ray diffraction and X-ray photoelectron spectroscopy that alloys and oxides are closely stacked and arranged alternately, and aluminate oxides induce strong electron interaction between Cu, Zn and Al, forming a large number of highly active reaction interfaces composed of 0 ~+3 valence metal sites.
The linear sweep voltammetric curve (LSV) of CuZn/CuZnAl2O4 catalyst showed that the cathode current increased sharply after CO2 was introduced. When CO2 was replaced by N2, the response of the current of CuZn/CuZnAl2O4 with voltage change decreased obviously, which indicated that CuZn/CuZnAl2O4 had higher CO2R activity and lower HER activity. At -1.15 V, CuZn/CuZnAl2O4 catalyst achieves the highest Faraday efficiency of 88.5% and the current density of C2+ reaches 360 mA/cm2. At this potential, the conversion of HER and CO2R to CO was inhibited, and the selectivity of H2 and C1 was 4.3% and 4.4% respectively.
In contrast, the selectivity of C2+ of Cu/CuAl2O4 catalyst is only 68.1% at -1.1 V, and the Faraday efficiency of C2+ decreases to 50.4% at -1.1 V of CuZn. At the same time, the C2+ current density of CuZn/CuZnAl2O4 catalyst is 2 times and 3 times higher than that of Cu/CuAl2O4 and CuZn catalyst, respectively. Compared with CuZn/CuZnAl2O4 catalyst, Cu/CuAl2O4 catalyst has the lowest FE content in CO, while CuZn catalyst has the highest FE content in CO, indicating that Zn element in the alloy accelerates the formation of CO. Moreover, CuZn alloy, CuZnAl2O4 oxide or their mixture all show very low C2+ Faraday efficiency, which indicates that the high activity in CuZn/CuZnAl2O4 comes from the tightly packed and strongly interacting alloy/oxide interface. Under the current density of 400 mA/cm2, the Faraday efficiency of C2+ products did not decrease obviously during the stability test of CuZn/CuZnAl2O4 catalyst for 10 h..
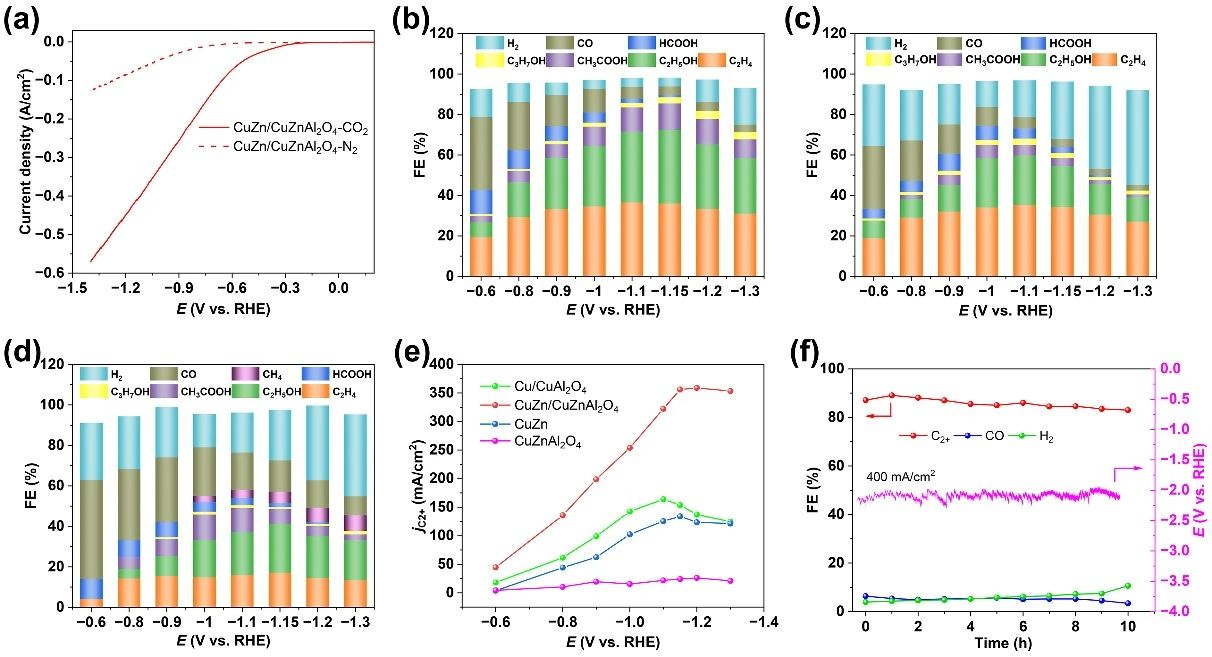
Fig. 2 Performance test of CO2R on CuZn/CuZnAl2O4, Cu/CuAl2O4 and CuZn catalysts. (a) LSV curve of CuZn/CuZnAl2O4 catalyst in CO2R, product distribution and corresponding Faraday efficiency of CO2R on CuZn/CuZnAl2O4(b), Cu/CuAl2O4(c) and CuZn(d), (e) current density of C2+product, (f)CuZn/CuZnAl2O4 in 2.
In-situ attenuated total reflection infrared spectroscopy (ATR-IRAS) was used to further study the synthesis of C2+ from CO2R on CuZn/CuZnAl2O4. In the infrared spectrum of CuZn/CuZnAl2O4, there are two strong absorption peaks, which are located at 1650 cm-1 and 1380 cm-1 respectively, belonging to H2O and *COOH species, indicating that the catalyst has strong adsorption and activation ability for H2O and CO2 molecules.
In addition, H2O and CO2 molecules are adsorbed and activated at the same time, indicating that there is a good coupling between H2O dissociation and CO2R, which can effectively inhibit HER. The activated *CO2 species and the surface *Had species will promote the generation of *CO species, which can be confirmed by the absorption peak of *CO appearing around 2068 cm-1 in the infrared spectrum. And the strong infrared absorption peak of *CO indicates that its surface has a high coverage of *CO; The comparative analysis shows that the metal-oxide interface improves the coverage of *CO, and it is further enhanced with the addition of Zn species.
Obviously, compared with CuZn/CuZnAl2O4 and CuZn catalysts, C2 intermediates * OCOH and *OC2H5 have the strongest infrared absorption peaks on CuZn/CuZnAl2O4, indicating that C2 intermediates have a stable adsorption structure on the catalyst surface, and CuZn alloy phase can accelerate the C-C coupling process, which is followed by the increase of C2+ product selectivity.
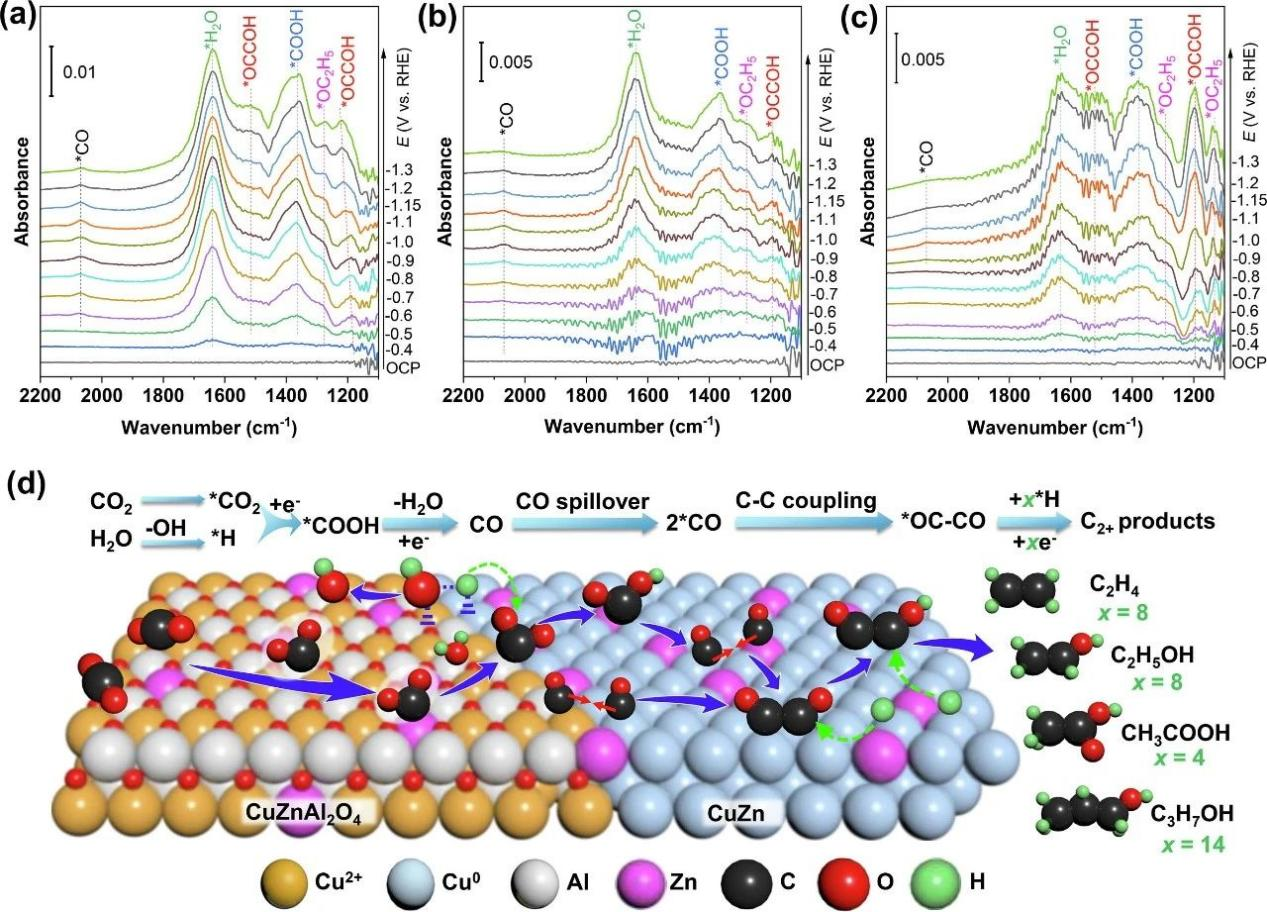
Fig. 3 In-situ ATR-IRAS test and reaction mechanism of catalyst under CO2R condition. (a)CuZn/CuZnAl2O4, (b)Cu/CuAl2O4 and (c)CuZn, and (d) the reaction mechanism of the reduction of CO2R to C2+ products at the interface of CuZn/CuZnAl2O4.
Source:
[1] Zhang Z.-Y., Tian H., Bian L., Liu S.-Z., Liu Y., Wang Z.-L.Cu-Zn-based alloy/oxide interfaces for enhanced electroreduction of CO2 to C2+ products
(2023) .Journal of Energy Chemistry[J], 90 - 97.
