Electrocatalytic CO2, cathode energy efficiency of 97.1%!
As a sustainable method, electrochemical CO2 reduction (ECR) has attracted much attention in CO2 conversion and carbon neutral economic cycle. At present, a great challenge of ECR is to improve energy efficiency, which requires improving Faraday efficiency (FE) and reducing overpotential. In recent years, the research shows that the cheap transition metal and nitrogen co-doped carbon (M-N-C) catalyst with atomic dispersed M-Nx sites have excellent performance in the process of ECR to CO, in which Fe-N-C electrocatalyst can achieve high FECO under the condition of low overpotential, but there is still much room for improvement.
Adjusting the coordination structure of central metal atoms can improve the selectivity and activity of M-N-C catalyst in ECR reaction. Thanks to the abundant metal sites and high binding energy, metal NPs can theoretically stabilize the intermediates in the reaction process, so introducing Fe NPs into Fe-N-C is expected to be an effective strategy to reduce the overpotential of ECR reaction. In addition, the oxygen components in various oxygen-containing groups on carbon carriers can not be ignored, which has been proved to affect the catalytic performance of M-N-C N-C.
Recently, Li Wei of Nankai University and others designed and synthesized a hybrid electrocatalyst consisting of Fe nanoparticles (Fe NPs), pyrrole-type Fe-N4 sites and carbon carriers with low oxygen content. The catalyst showed remarkable CO Faraday efficiency (FECO) of over 99% at ultra-low overpotential of 21 mV, reaching the highest cathode energy efficiency of 97.1% so far. In addition, it can provide CO selectivity close to 100% and high cathode energy efficiency (> 90%) of at least 100 h.. The related work was published in the internationally renowned journal Nature Communications with the title of "Combining Fe nanoparticles and Pyrroletype Fe-N4 sites on less-oxygenated carbon supports for electrochemical CO2 reduction".
Key point 1. The author has prepared two kinds of modified Fe-N-C electrocatalysts with Fe NPs and pyrrole type Fe-N4 sites (Fe-poN-C/Fe and Fe-poN-C/(O)) fixed on low-oxygen carbon substrates and oxygen-containing carbon substrates.
Key point 2. The results show that Fe-poN-C/Fe can provide 99.7% high FECO at a low overpotential of 0.24 V in H-type battery, and it shows 97.1% ultra-high cathode energy efficiency (CEE), nearly 100% FECO and 14.1 macm-2 current at an ultra-low overpotential of 21 mV in mobile battery. In addition, the stability test over 100 hours shows that Fe-poN-C/Fe can produce high CEE(>90%) and nearly 100% CO selectivity when the current density exceeds 40 macm-2.
Key point 3. The comprehensive results of control experiment, in-situ characterization and theoretical calculation show that the introduction of Fe nanoparticles can reduce the overpotential by accelerating the proton transfer from CO2 to *COOH and reducing the free energy for the formation of *COOH. The construction of pyrrole-type Fe-N4 sites on carbon carriers and carbon carriers with less oxygen can improve FECO by inhibiting the precipitation of H2, thus realizing energy-saving electrochemical reduction of CO2 to CO..
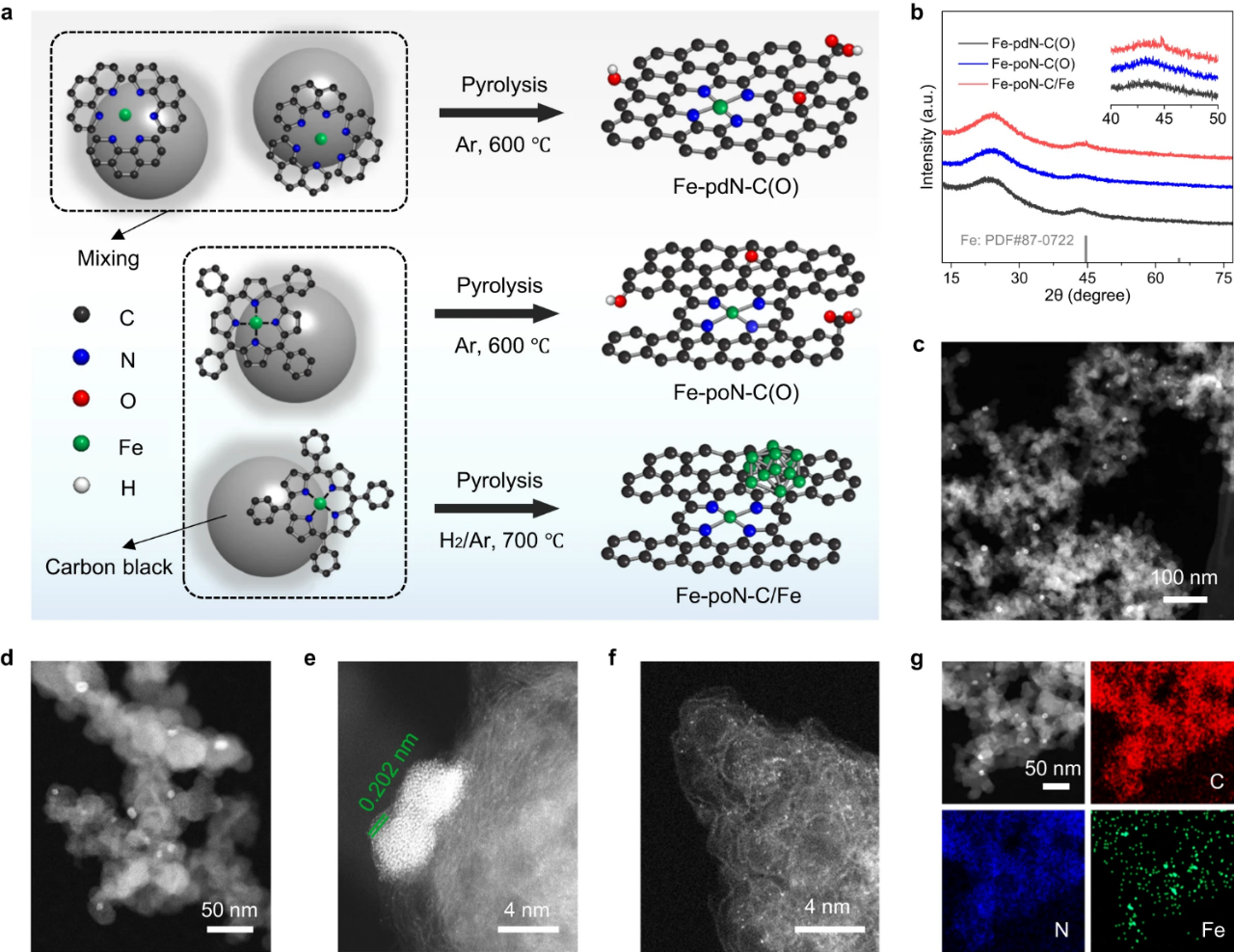
Fig. 1. (a) Schematic diagram of the synthesis process of Fe-PDN-C (o), Fe-PON-C (o) and Fe-poN-C/Fe. (b) XRD diffraction of Fe-PDN-C (o), Fe-PON-C (o) and Fe-poN-C/Fe. (c) low magnification HAADF-TEM diagram, (d) high magnification HAADF-TEM diagram, (e,f) aberration corrected HAADF-STEM diagram and (g)EDS element map of Fe-poN-C/Fe.
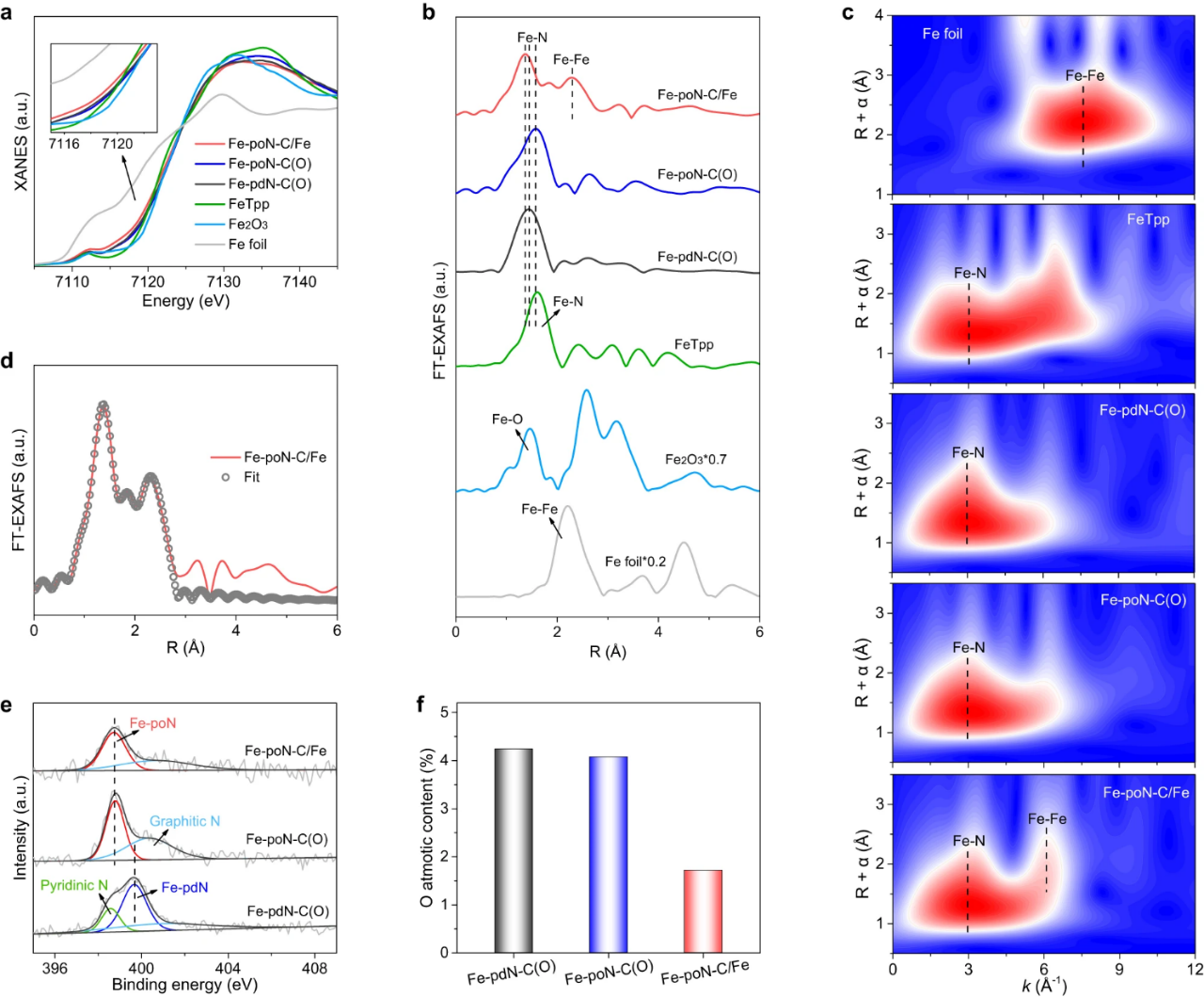
Fig. 2. (a) Fe K-edge XANES spectra and (b) Fe K-edge FT-EXAFS spectra of Fe-PDN-C (o), Fe-PON-C (o) and Fe-poN-C/Fe and control samples. (c) WT-EXAFS analysis of Fe foil, FeTpp, Fe-pdN-C(O), Fe-poN-C(O) and Fe-poN-C/Fe. (d) FT-EXAFS fitting curve of Fe-PON-C/Fe. (e)N 1s spectra and (f)O atomic content of Fe-pdN-C(O), Fe-poN-C(O) and Fe-poN-C/Fe.
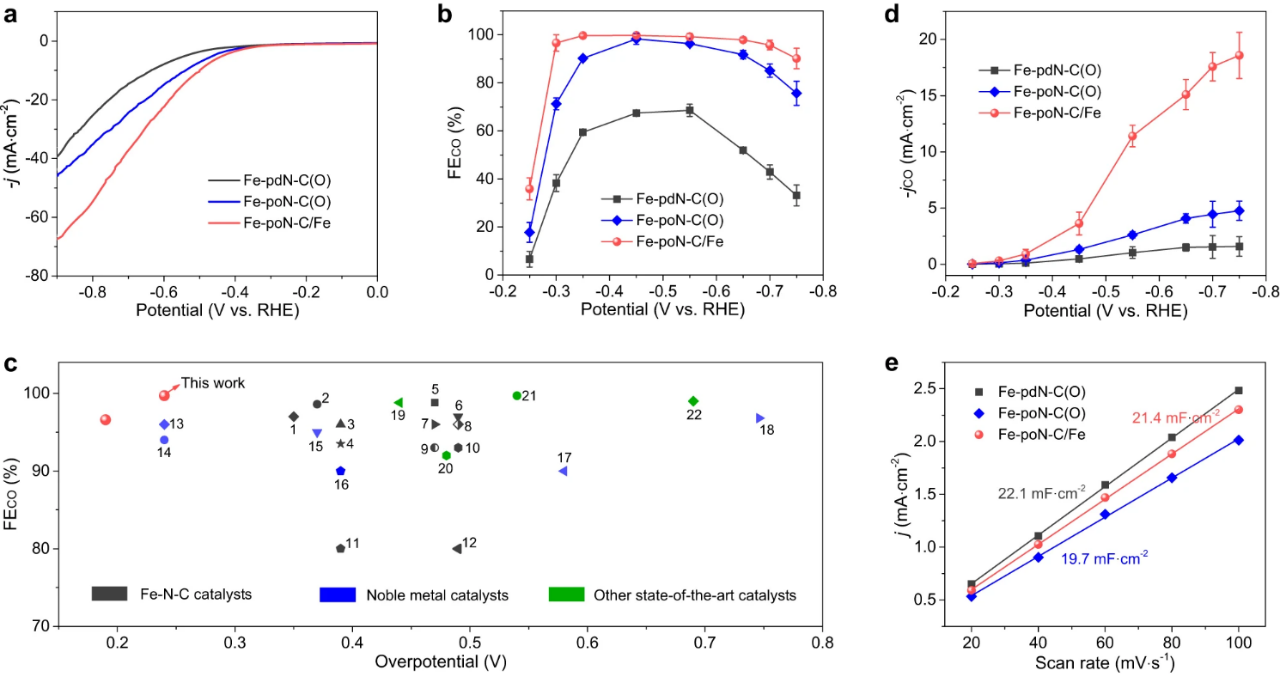
Fig. 3. Electrochemical CO2 reduction performance in H-cell. (a) LSV curves of Fe-PDN-c (o), Fe-pon-c (o) and Fe-poN-C/Fe. (b) CO Faraday efficiencies of Fe-PDN-C (o), Fe-PON-C (o) and Fe-poN-C/Fe under various applied potentials. (c) The ECR performance of Fe-PON-C/Fe is compared with other electrocatalysts previously reported. (d) jCO of Fe-PDN-C (o), Fe-PON-C (o) and Fe-poN-C/Fe at various applied potentials. (e) Electric double layer capacitance of Fe-PDN-C (o), Fe-PON-C (o) and Fe-poN-C/Fe.
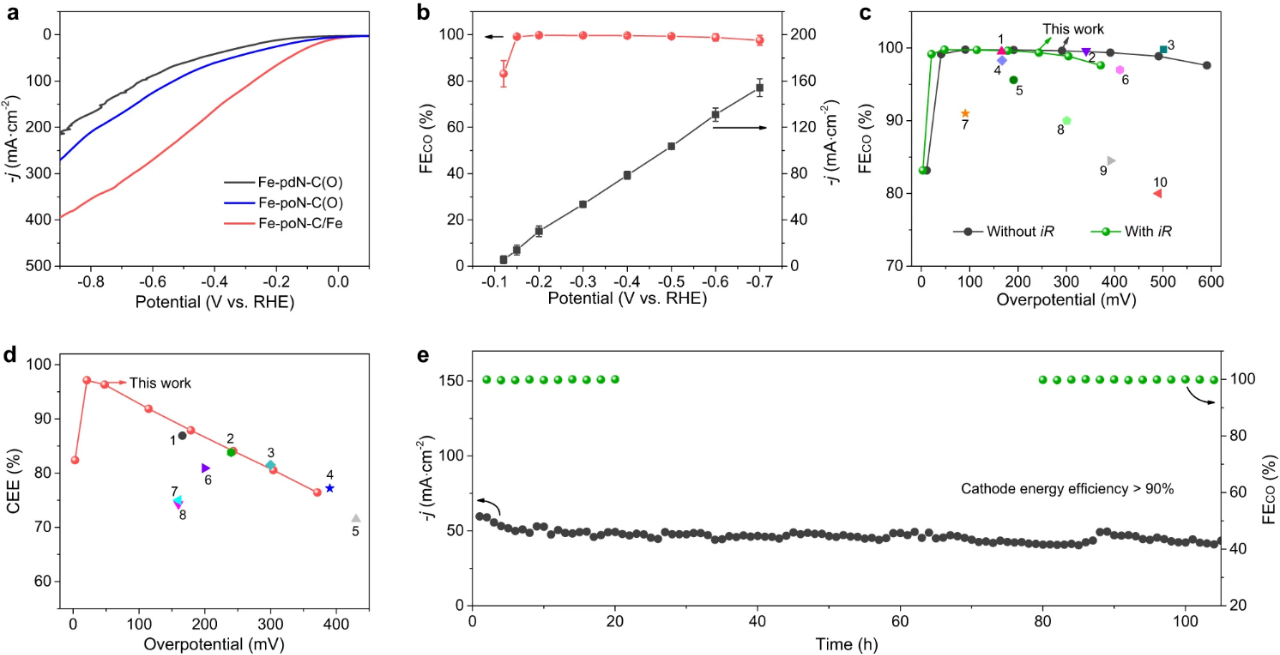
Fig. 4. Electrochemical CO2 reduction performance in a flow cell. (a) LSV curves of Fe-PDN-c (o), Fe-pon-c (o) and Fe-poN-C/Fe. (b) FECO and corresponding J of Fe-PON-C/Fe at various potentials. The (c) FECO and (d) CEE values of Fe-poN-C/Fe were compared with those of other electrocatalysts previously reported. (e) the stability test of Fe-pon-c/Fe at an applied potential of 0.3 v.
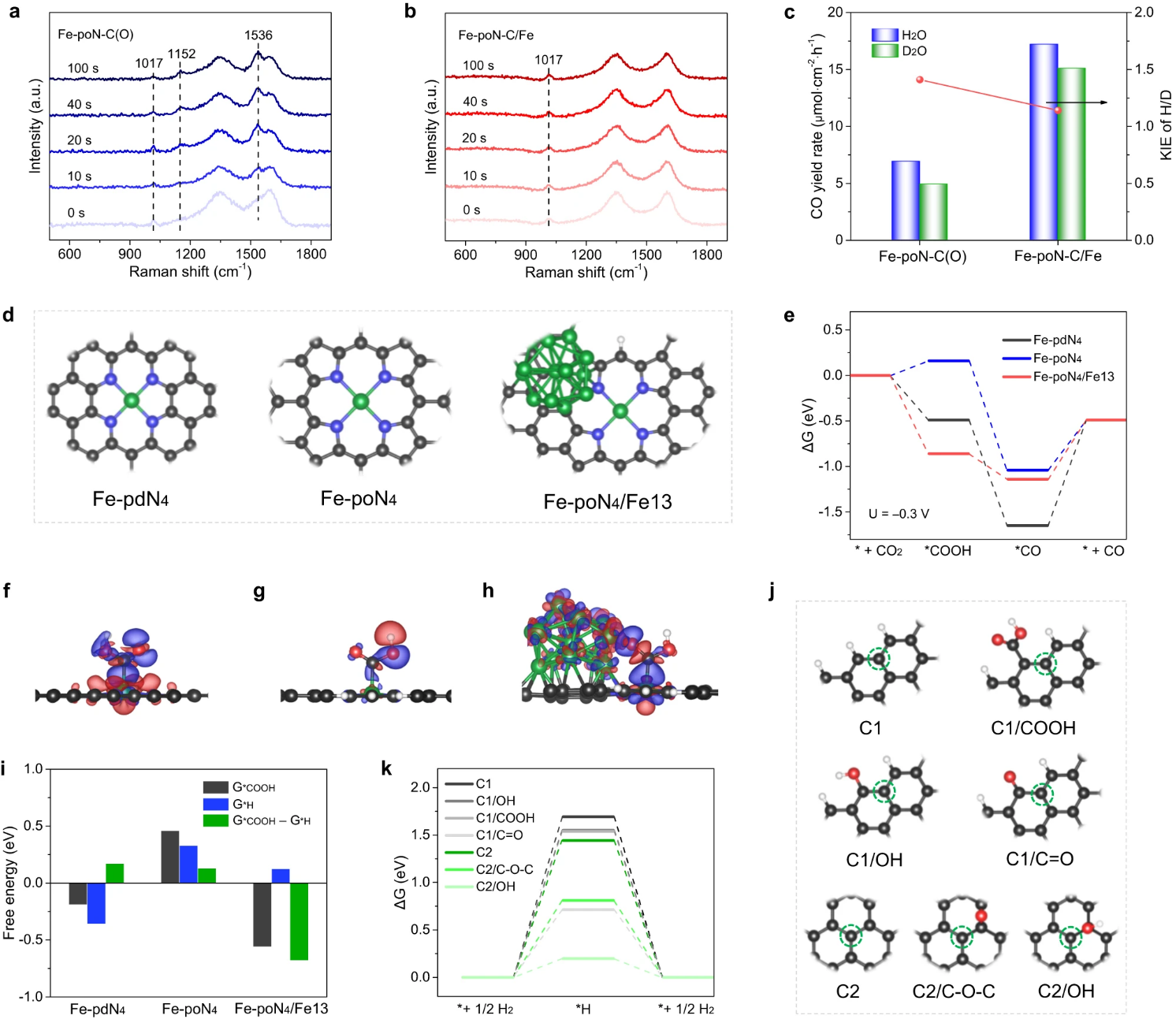
Fig. 5. In-situ Raman spectra of (a) Fe-PON-C (o) and (b) Fe-poN-C/Fe. (c) KIE value and CO yield of Fe-PON-C (O) and Fe-poN-C/Fe. (d) Optimized structures of Fe-PDN 4, Fe-PON4 and Fe-poN4/Fe13. (e) Free energy of ECR process at −0.3 V potential. (f) Differential charge densities of Fe-PDN 4, (g) Fe-PON4 and (h) Fe-poN4/Fe13 after adsorption of *COOH. (i) The formation free energy of * i)*COOH (G*COOH) or *H (G*H), and the difference between G*COOH and G*H. (j) Optimized graphene structures with different oxygen-containing groups. (k) HER free energy of graphene structures with different oxygen-containing groups.
Source:https://mp.weixin.qq.com/s/oVYCfus6zoTVUdH6hO7i4w