Photocatalytic CO2 to CO, the selectivity is nearly 100%, and the yield is increased by 20 times!
In recent years, bismuth-based semiconductors have attracted extensive attention in photocatalytic carbon dioxide emission reduction because of their unique electronic structure, appropriate band gap, adjustable surface structure and non-toxic properties. However, the original Bi2Sn2O7 photocatalyst has low conversion activity for CO2 reduction, which is mainly due to high charge recombination and lack of catalytic active sites. On the contrary, the reasonable design and construction of space-limited sub-nano metal clusters in various carriers has been widely studied as a promising strategy to achieve high activity and selectivity in thermal catalysis and electrocatalytic reactions.
Recently, the team of Bi Yingpu, a researcher from Lanzhou Institute of Chemical Physics, Chinese Academy of Sciences, reported a simple method to construct sub-nano Bi metal clusters with limited space in situ in Bi2Sn2O7, thus significantly enhancing the photocatalytic CO2 reduction driven by solar energy. These confined Bi clusters promote charge separation and electron enrichment, and provide catalytic active sites for CO2 adsorption/activation of CO formation. Photocatalytic activity and selectivity of CO2 reduction to CO were significantly improved, and the yield of CO was as high as 114.1 μ mol-1h-1, which was more than 20 times that of the original Bi2Sn2O7. Reasonable construction of space-limited metal clusters in photocatalyst should be a promising strategy to effectively promote carbon dioxide emission reduction activities.
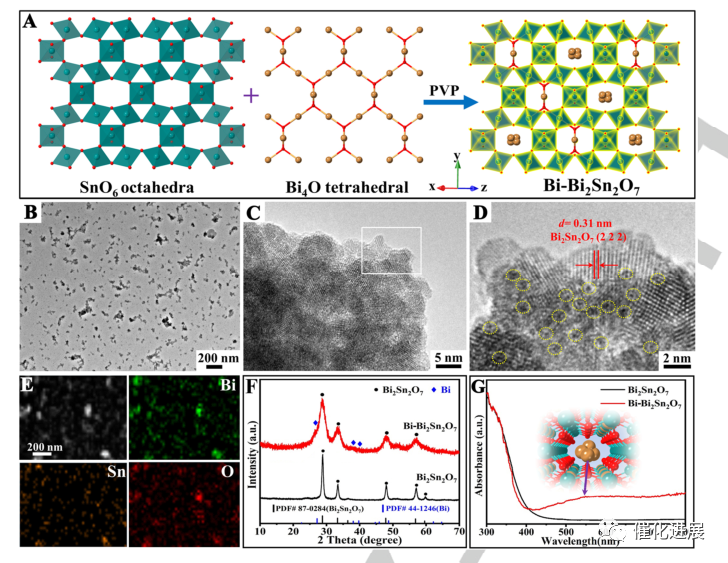
Fig. 1 preparation route and its physical and chemical characterization
Using PVP as reducing agent, the space-limited sub-nano Bi clusters in Bi2Sn2O7 photocatalyst (Bi-Bi2Sn2O7) were synthesized by one-step hydrothermal method. The obtained Bi-Bi2Sn2O7 nanocrystals have uniform diameter and high dispersibility, and metallic Bi clusters with a diameter of about 0.43 nm are uniformly dispersed on the crystal lattice. Due to the in-situ transformation of Bi4O tetrahedron into metallic Bi clusters, the crystallinity of Bi2Sn2O7 decreased, which further proved the limited Bi clusters in Bi2Sn2O7 photocatalyst. The confinement effect of sub-nano Bi clusters can effectively enhance the absorption of visible light in the wavelength range of 400-800 nm, which should be attributed to the unique plasma effect of confined Bi metal clusters.
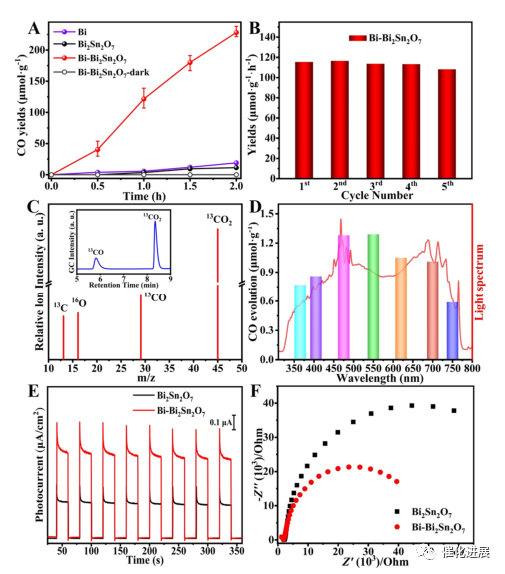
Fig. 2 Photocatalytic performance
Subsequently, the photocatalytic performance of Bi-Bi2Sn2O7 was evaluated under simulated sunlight. Bi-Bi2Sn2O7 showed excellent photocatalytic activity. After 2 hours, the CO content was as high as 228.2 μ mol-1, which was much higher than that of original Bi2Sn2O7 and metallic Bi. The selectivity of CO2 reduction to CO was close to 100%, and no other carbon-containing products were detected. Cyclic experiments show that the photocatalyst also shows excellent stability and structural stability. Isotope tracing experiments confirmed that the formed CO was from photocatalytic CO2 conversion. Photocatalytic CO precipitation activity was observed in the whole wavelength range of 300-800 nm. Restricted Bi clusters not only promote the photoreduction activity of CO2, but also increase the utilization rate of sunlight. Photoelectrochemical measurements show that Bi-Bi2Sn2O7 has higher photocurrent density and better charge transfer ability compared with the original Bi2SN2O7. These results show that the confined sub-nano Bi clusters can effectively promote charge separation and migration, thus achieving high CO2 reduction activity.
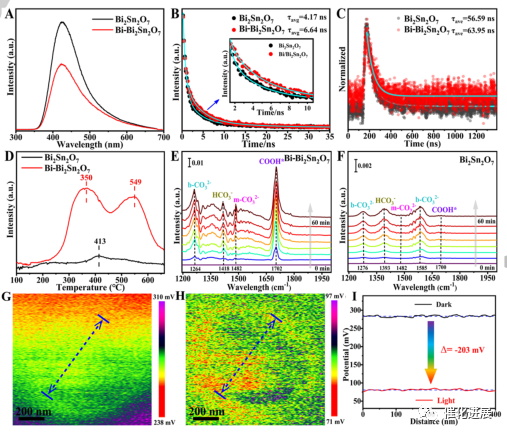
Fig. 3 explanation of reaction mechanism
PL, TR-PL and TR-TAS aim to explore the influence of restricted Bi clusters in Bi2Sn2O7. The formation of metallic Bi clusters in Bi2Sn2O7 reduces the peak intensity of PL, which indicates that the inhibition of photogenerated electron holes is more effective for recombination. The average carrier lifetime of Bi-Bi2Sn2O7 is much longer than that of the original Bi2Sn2O7, and compared with Bi2Sn2O7, Bi-Bi2Sn2O7 has higher absorption peak and longer carrier lifetime. The introduction of metallic Bi clusters significantly promoted the chemical adsorption of CO2 molecules, and the related electrochemical results can further support this point.
In-situ FTIR spectra show that the surface of Bi-Bi2Sn2O7 provides reaction sites more effectively, which promotes the formation of *COOH intermediates and the subsequent protonation of *CO to form CO, which promotes CO2 activation vibration due to strong COO stretching. The results of CO2-TPD and in-situ FTIR show that Bi-Bi2Sn2O7 exhibits higher adsorption and activation capacity for CO2 molecules than the original Bi2Sn2O7 sample.
Quasi-in-situ X-ray photoelectron spectroscopy (QIS-XPS) was used to analyze the electronic structure and chemical state of the surface atoms of Bi-Bi2Sn2O7 during CO2 adsorption and activation. CO2 adsorption and dissociation were observed in C 1s spectrum, which were represented by *CO2 and *CO peaks. The Sn 3d peak did not show significant changes. Under light irradiation, the intensity of *CO2 peak decreases, while the intensity of *CO peak increases, indicating that CO2 is further adsorbed/dissociated on the surface of Bi-Bi2Sn2O7. Bi-Bi peak is enhanced and moved to the lower binding energy position, indicating that electrons are enriched in metal Bi clusters. No significant change was observed in Sn 3d peak. These results confirm the key role of Bi metal clusters in the adsorption/activation of CO2 molecules.
Based on these findings, a possible mechanism is proposed, in which metal Bi clusters with electron-rich spatial constraints promote CO2 absorption and subsequent protonation of *CO2 under light irradiation to form *COOH intermediates. Then, the *COOH intermediate is reduced by proton and electron transfer, resulting in *CO desorption and CO formation. The existence of Bi metal clusters in Bi2Sn2O7 provides an effective reaction channel for photocatalytic reduction of CO2 to CO.
Source:https://mp.weixin.qq.com/s/qJ16LP5mw0pWRhVIMaoETw
