Dialectical Effects of MoO3 Modification on Pt/Al2O3 for VOC Combustion: Molecular-Structure-Sensitive Insights
Press-ready text
A research team led by Lin Dong at Nanjing University has reported a systematic study showing that “acid modification” is not a one-size-fits-all strategy for improving Pt-based catalysts in VOC catalytic combustion. Published online ahead of print in Environmental Science & Technology (Dec 27, 2025; DOI: 10.1021/acs.est.5c12397), the paper demonstrates that the benefit—or penalty—of acidity enhancement depends strongly on the molecular structure of the target VOC, especially whether it contains pi bonds.
Why it matters
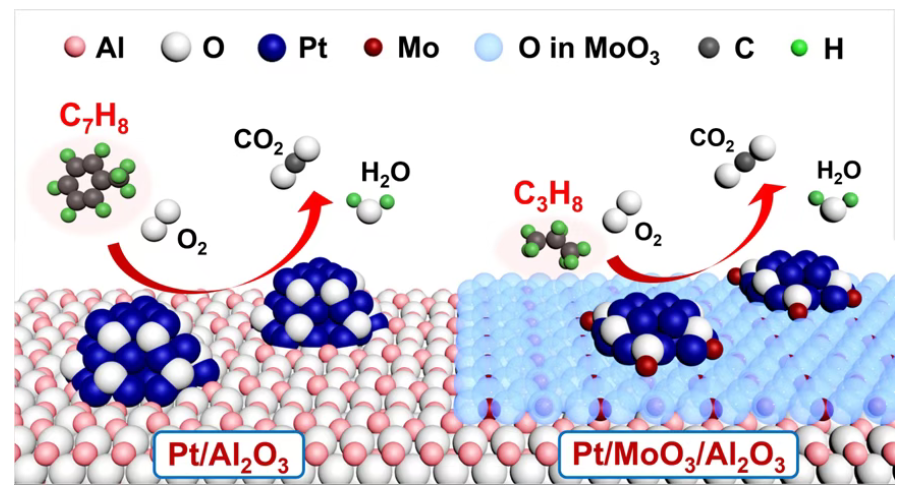
Catalytic combustion is widely used to remove VOCs from industrial exhaust, and Pt/Al2O3 is a benchmark noble-metal catalyst platform. In recent years, acid modification (e.g., introducing acidic oxides) has been frequently adopted to “boost activity.” However, the field has paid comparatively less attention to how the same modification may behave differently across VOC families. This work addresses that gap by explicitly linking the modification effect to VOC molecular structure.
What the team found: a clear “dialectical effect”
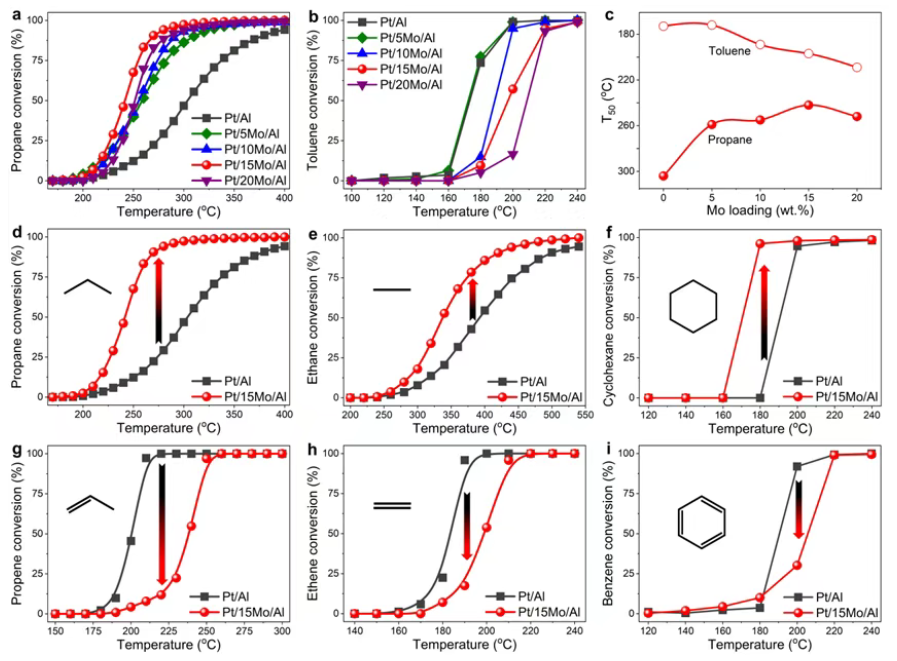
Using MoO3-modified Pt/Al2O3 as a model catalyst series, the authors selected VOC probes with distinct structural features and observed a striking split:
For weakly adsorbing saturated alkanes (e.g., propane, cyclohexane), MoO3 modification enhances combustion performance. The improved surface acidity and a higher fraction of metallic Pt sites help alleviate alkane–O2 competitive adsorption, promote C–H activation, and accelerate the decomposition/desorption of reaction intermediates—collectively raising the overall oxidation rate.
For VOCs containing pi bonds (e.g., toluene, propene), the same MoO3 modification becomes detrimental. Stronger adsorption of pi-bond VOCs on the acid-modified surface suppresses deep oxidation pathways, leading to inferior reactivity and, in practice, a higher risk of intermediate accumulation and carbon deposition under combustion conditions.
How the mechanism was supported (key experimental logic)
Beyond activity tests, the study combines structural and surface analyses to explain why MoO3 changes performance in opposite directions. The authors report highly dispersed Pt species across the catalyst series, and they show that MoO3 increases the number and strength of surface acid sites (via NH3-TPD and pyridine-IR). To connect these properties to reaction pathways, they further used in situ DRIFTS with propane and toluene as probe reactions. The spectroscopy results support that MoO3 modification enables earlier/lower-temperature activation of alkanes and faster conversion of reactive intermediates toward CO2, whereas pi-bond VOCs become over-stabilized on the surface, hindering complete oxidation and favoring intermediate buildup.
Design takeaway
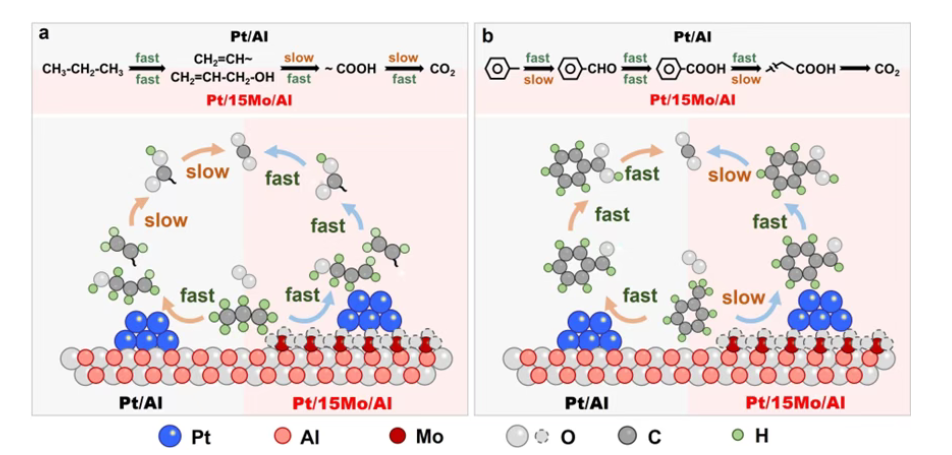
The main message is practical: “more acidity” is not automatically better for Pt-catalyzed VOC combustion. Catalyst design should start from the target VOC portfolio. For alkane-rich exhaust streams, acidity enhancement (such as MoO3) can be beneficial by improving adsorption/activation and easing competition with oxygen. For streams dominated by aromatic or olefinic VOCs, excessive acidity can backfire by over-strengthening adsorption and suppressing deep oxidation. The authors also note that similar structure-sensitive trends are observable across related Pt-based systems, suggesting a broadly applicable design principle rather than an isolated case.
